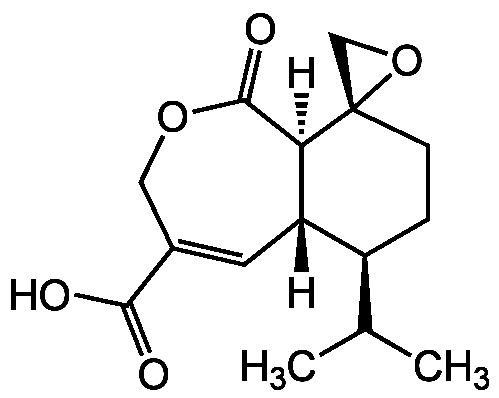
Chemical Structure
Heptelidic acid [57710-57-3] [57710-57-3]
AG-CN2-0118
CAS Number57710-57-3
Product group Chemicals
Estimated Purity>95%
Molecular Weight280.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameHeptelidic acid [57710-57-3] [57710-57-3]
- Delivery Days Customer10
- CAS Number57710-57-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC15H20O5
- Molecular Weight280.3
- Scientific DescriptionAntibiotic [1, 2]. Potent selective glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inhibitor. Binds to the essential Cys149 residue in the catalytic site of GAPDH [3-7, 10, 11]. Anticancer compound [8]. Selectively kills high-glycolytic cancer cells through glucose-dependent active ATP deprivation [12]. Antimalarial [9]. Apoptosis inhibitor. DNA fragmentation and caspase-3 activation inhibitor [13,16]. Selective and competitive inhibitor of mammalian DNA polymerases beta, lambda and terminal deoxynucleotidyl transferase (TdT) in family X of pols [14]. Reduces lactate secretion and causes reductions in overall protein synthesis and production of IFN-alpha and TNF. - Chemical. CAS: 57710-57-3 (74310-84-2 deleted). Formula: C15H20O5. MW: 280.3. Isolated from Trichoderma sp. Antibiotic. Potent selective glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inhibitor. Binds to the essential Cys149 residue in the catalytic site of GADPH. Anticancer compound. Selectively kills high-glycolytic cancer cells through glucose-dependent active ATP deprivation. Antimalarial. Apoptosis inhibitor. DNA fragmentation and caspase-3 activation inhibitor. Selective and competitive inhibitor of mammalian DNA polymerases beta, lambda and terminal deoxynucleotidyl transferase (TdT) in family X of pols.
- SMILES[H][C@@]12C=C(COC(=O)[C@@]1([H])[C@@]1(CO1)CC[C@H]2C(C)C)C(O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

