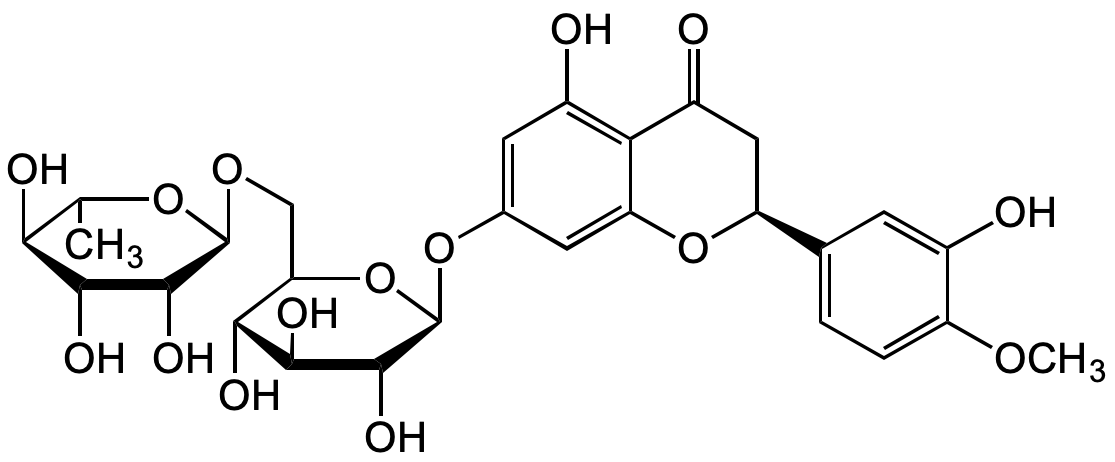
Chemical Structure
Hesperidin
CDX-H0245
CAS Number520-26-3
Product group Chemicals
Estimated Purity>98%
Molecular Weight610.56
Overview
- SupplierChemodex
- Product NameHesperidin
- Delivery Days Customer10
- CAS Number520-26-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC28H34O15
- Molecular Weight610.56
- Scientific DescriptionChemical. CAS: 520-26-3. Formula: C28H34O15. MW: 610.56. Isolated from plant source. Hesperidin is a flavanone rutinoside first isolated from citrus peels. It is metabolized by intestinal bacteria to an aglycone form, hesperetin, which is thought to be more bioavailable due to reduced polarity that allows for increased cell permeability. Both hesperidin (Hsd) and its aglycone hesperetin (Hst) have various biological properties. Studies have shown both anti-cancer and cancer chemopreventive effects, associated with their antioxidant, radical scavenging and anti-inflammatory activities. In addition, Hsd and Hst interfere at different stages of cancer. They inhibit tumor growth by targeting multiple cellular protein targets at the same time, including caspases, Bcl-2 and Bax for the induction of apoptosis, and COX-2, MMP-2 and MMP-9 for the inhibition of angiogenesis and metastasis. They also have show cardioprotective, neuroprotective and antimicrobial effects. - Hesperidin is a flavanone rutinoside first isolated from citrus peels. It is metabolized by intestinal bacteria to an aglycone form, hesperetin, which is thought to be more bioavailable due to reduced polarity that allows for increased cell permeability. Both hesperidin (Hsd) and its aglycone hesperetin (Hst) have various biological properties. Studies have shown both anti-cancer and cancer chemopreventive effects, associated with their antioxidant, radical scavenging and anti-inflammatory activities. In addition, Hsd and Hst interfere at different stages of cancer. They inhibit tumor growth by targeting multiple cellular protein targets at the same time, including caspases, Bcl-2 and Bax for the induction of apoptosis, and COX-2, MMP-2 and MMP-9 for the inhibition of angiogenesis and metastasis. They also have show cardioprotective, neuroprotective and antimicrobial effects. Antiviral, potential inhibitor against SARS-CoV-2 infection.
- SMILESO=C1C[C@@H](C2=CC=C(OC)C(O)=C2)OC3=CC(O[C@H]4[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@H]5[C@H](O)[C@H](O)[C@@H](O)[C@H](C)O5)O4)=CC(O)=C31
- Storage Instruction2°C to 8°C
- UNSPSC12352200

![Hesperidin [520-26-3]](https://www.targetmol.com/group3/M00/02/F8/CgoaEGY7QyCEIgwpAAAAADaO5rI208.png)
