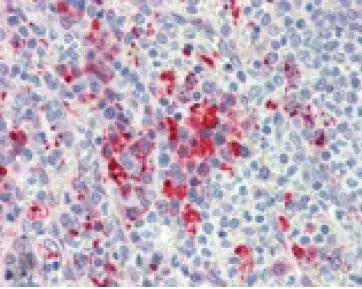
Immunohistochemistry(paraffin sections) analysis of human spleen tissue stained with HSP90, mAb (AC88) at 10microg/ml.
Hsp90 antibody [AC88]
GTX13492
ApplicationsFlow Cytometry, ImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
Product group Antibodies
ReactivityBovine, Canine, Fish, Guinea Pig, Hamster, Human, Monkey, Mouse, Porcine, Rabbit, Rat, Sheep
Overview
- SupplierGeneTex
- Product NameHsp90 antibody [AC88]
- Delivery Days Customer9
- Antibody SpecificityGTX13492 recognises both Hsp90 alpha and beta. It reacts with the rodent glucocorticoid receptor and with proliferation potential proteins (P2PS) with apparent molecular masses of 30-40 kDa, which are associated with the 30-40S substructures of nuclear hn
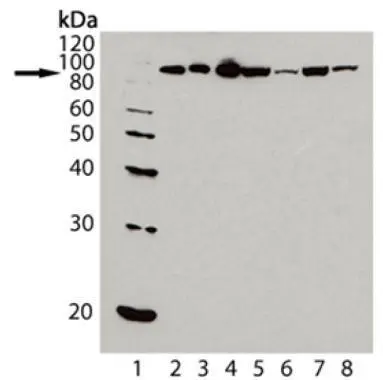
- Application Supplier NoteIHC-P: Use at an assay dependent dilution.When used in immunoprecipitation, this antibody does not appear to react well with Hsp90 that is bound to steroid receptors or to pp60v-src of Rouse sarcoma virus. WB: Use at a concentration of 1 microg/ml. Detects a band of approximately 90 kDa (predicted molecular weight: 84.7 kDa(alpha) and 83.2 kDa (beta)). Optimal dilutions/concentrations should be determined by the end user.
- ApplicationsFlow Cytometry, ImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDAC88
- Concentration1 mg/ml
- ConjugateUnconjugated
- HostMouse
- IsotypeIgG1
- Scientific DescriptionThe 90kDa molecular chaperone family comprises several proteins including the 90kDa heat shock protein, Hsp90 and the 94kDa glucose regulated protein, grp94 which are major molecular chaperones of the cytosol and of the endoplasmic reticulum. In mammalian cells there are at least two Hsp90 isoforms, Hsp90a and hsp90s which are encoded by separate genes. The amino acid sequence of human and yeast Hsp90a is 85% and 90% homologous to that of Hsp90s respectively. All known members of the Hsp90 protein family are highly conserved, especially in the N terminal and C terminal regions which have been shown to contain independent chaperone sites with different substrate specificity. These ubiquitous and highly conserved proteins account for 1-2% of all cellular proteins in most cells. Hsp90 is part of the cells powerful network of chaperones to fight the deleterious consequences of protein unfolding caused by nonphysiological conditions. However, in the absence of stress, Hsp90 is a necessary component of fundamental cellular processes such as hormone signaling and cell cycle control. In this context several key regulatory proteins such as steriod receptors, cell cycle kinases involved in signal transduction and p53 have been identified as substrates of Hsp90. It has been suggested that Hsp90 acts as a capacitor for morphological evolution by buffering widespread variation, which may affect morphogenic pathways.
- ReactivityBovine, Canine, Fish, Guinea Pig, Hamster, Human, Monkey, Mouse, Porcine, Rabbit, Rat, Sheep
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC12352203
References
- CAG repeat expansion in the Huntingtons disease gene shapes linear and circular RNAs biogenesis.Read more