Iso-Insulin ELISA
10-1128-01
Product group Assays
Overview
- SupplierMercodia
- Product NameIso-Insulin ELISA
- Delivery Days Customer5
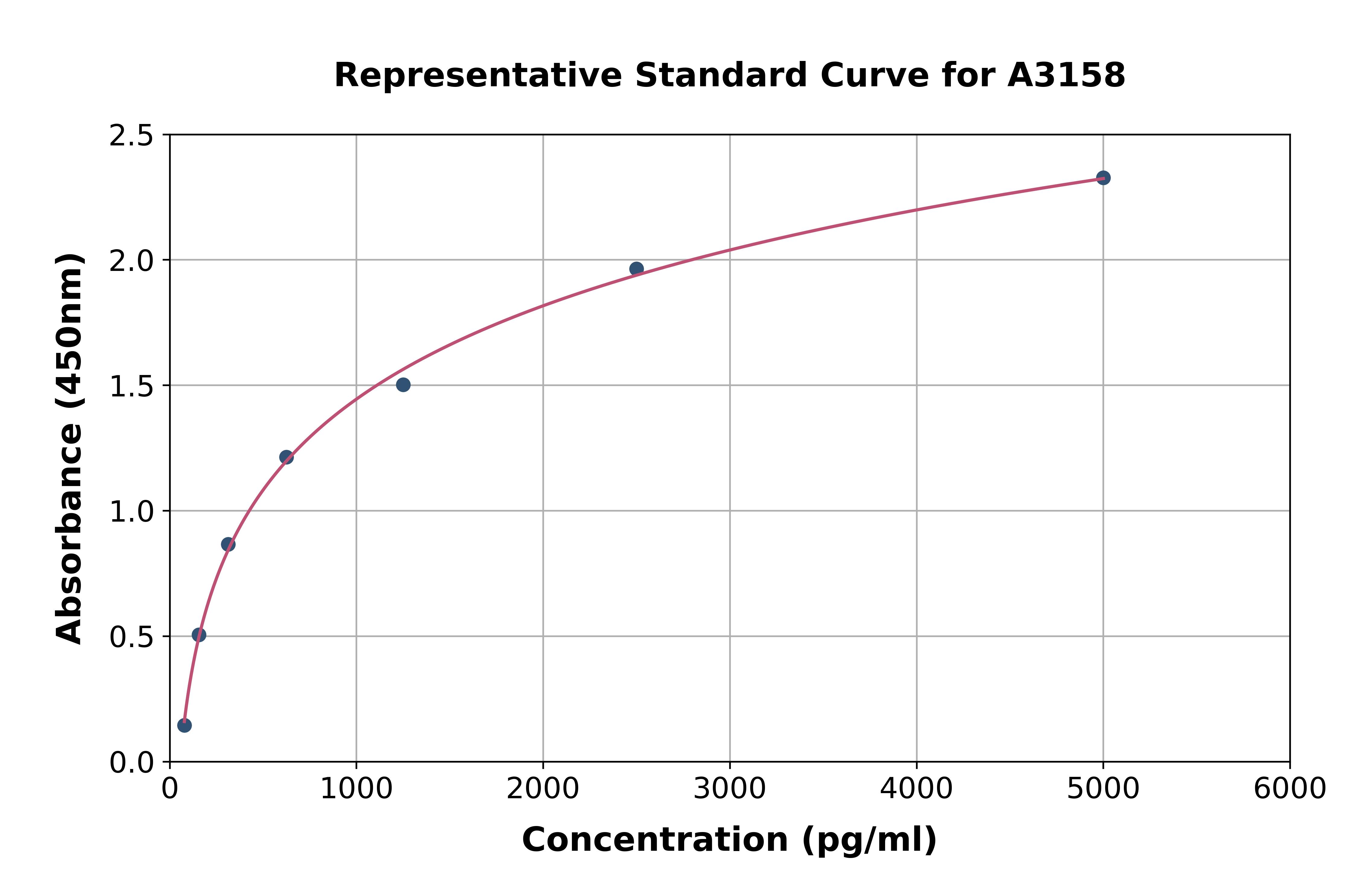
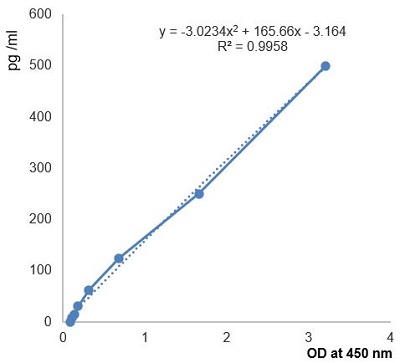
- ApplicationsELISA
- Assay Detection Range3 - 100 mU/L
- CertificationCE-IVD
- Scientific DescriptionMercodia Iso-Insulin ELISA provides a method for the quantitative determination of total insulin. It is a high quality enzyme immunoassay for the quantification of insulin; both endogenous and exogenous. The assay measures common, as well as non-commercially available, insulin analogues and comes with full cross-reactivity data. Combination of Mercodia Iso-Insulin ELISA with the highly specific Mercodia Insulin ELISA enables calculation of analog concentrations by subtracting endogenous insulin. Mercodia Diabetes Antigen Control (10-1134-01 (US only) or 10-1164-01) is designed to be used as a two-level control for human insulin. • Measures total insulin • Cross-reactivity with most insulin analogs • Calibrated against 1st International Reference Preparation 66/304 for human insulin. • CE/IVD labeled on select markets
- UNSPSC41116133
References
- Ramos-Rodriguez JJ, Sanchez-Sotano D, Doblas-Marquez A, et al. Intranasal insulin reverts central pathology and cognitive impairment in diabetic mother offspring. Mol Neurodegener. 2017,12(1):57. doi: 10.1186/s13024-017-0198-4Read this paper
- van Dijk PR, Logtenberg SJ, Chisalita SI, et al. Different Effects of Intraperitoneal and Subcutaneous Insulin Administration on the GH-IGF-1 Axis in Type 1 Diabetes. J Clin Endocrinol Metab. 2016,101(6):2493-501. doi: 10.1210/jc.2016-1473Read this paper
- Cengiz E, Swan KL, Tamborlane WV, et al. The alteration of aspart insulin pharmacodynamics when mixed with detemir insulin. Diabetes Care. 2012,35(4):690-2. doi: 10.2337/dc11-0732Read this paper
- Cengiz E, Tamborlane WV, Martin-Fredericksen M, et al. Early pharmacokinetic and pharmacodynamic effects of mixing lispro with glargine insulin: results of glucose clamp studies in youth with type 1 diabetes. Diabetes Care. 2010,33(5):1009-12. doi: 10.2337/dc09-2118Read this paper
- Sintov AC, Levy HV, Botner S. Systemic delivery of insulin via the nasal route using a new microemulsion system: In vitro and in vivo studies. J Control Release. 2010,148(2):168-76. doi: 10.1016/j.jconrel.2010.08.004Read this paper