Chemical Structure
Hymenidin [107019-95-4]

AG-CN2-0503
CAS Number107019-95-4
Product group Chemicals
Estimated Purity>97%
Molecular Weight310.2 . 18.0
Overview
- SupplierAdipoGen Life Sciences
- Product NameHymenidin [107019-95-4]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number107019-95-4
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationDanger,Excepted quantity
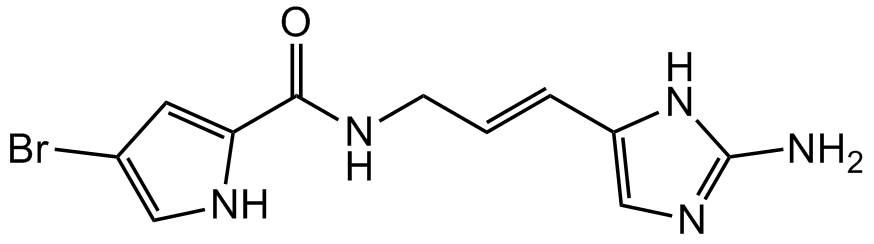
- Molecular FormulaC11H12BrN5O . H2O
- Molecular Weight310.2 . 18.0
- Scientific DescriptionChemical. CAS: 107019-95-4. Formula: C11H12BrN5O . H2O. MW: 310.2 . 18.0. Isolated from sponge Stylissa sp. Inhibitor of CDK5/p25 (IC50=4microM) and GSK-3beta (IC50=12microM). Potentially useful in neuronal diseases research. Antagonist of serotonergic receptors. Shown to reduce voltage-dependent calcium elevation. Moderate anticancer compound. Inhibits cell growth in a panel of cancer cell lines at low microM range. Antiprotozoal agent. Antibacterial. Plays a role in the sponge antibacterial defense. - Inhibitor of CDK5/p25 (IC50=4microM) and GSK-3beta (IC50=12microM). Potentially useful in neuronal diseases research. Antagonist of serotonergic receptors. Shown to reduce voltage-dependent calcium elevation. Moderate anticancer compound. Inhibits cell growth in a panel of cancer cell lines at low microM range. Antiprotozoal agent. Antibacterial. Plays a role in the sponge antibacterial defense.
- SMILESO=C(NC/C=C/C1=CN=C(N)N1)C2=CC(Br)=CN2
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 3462
- UNSPSC12352200
References
- A novel antagonist of serotonergic receptors, hymenidin, isolated from the Okinawan marine sponge Hymeniacidon sp: J. Kobayashi, et al.; Experientia 42, 1176 (1986)
- Inhibition of cyclin-dependent kinases, GSK-3beta and CK1 by hymenialdisine, a marine sponge constituent: L. Meijer, et al.; Chem. Biol. 7, 51 (2000)
- Brominated pyrrole alkaloids from marine Agelas sponges reduce depolarization-induced cellular calcium elevation: U. Bickmeyer, et al.; Toxicon 44, 45 (2004)
- Marine compounds for the therapeutic treatment of neurological disorders: D. Alonso, et al.; Expert Opin. Ther. Pat. 15, 1377 (2005)
- Antineoplastic agents 470. Absolute configuration of the marine sponge bromopyrrole agelastatin A: G.R. Pettit, et al.; Oncol. Res. 15, 11 (2005)
- Bromopyrrole alkaloids as lead compounds against protozoan parasites: F. Scala, et al.; Mar. Drugs 8, 2162 (2010)
- Chemical defense against predators and bacterial fouling in the Mediterranean sponges Axinella polypoides and A. verrucosa: M. Haber, et al.; MEPS 422, 113 (2011)
