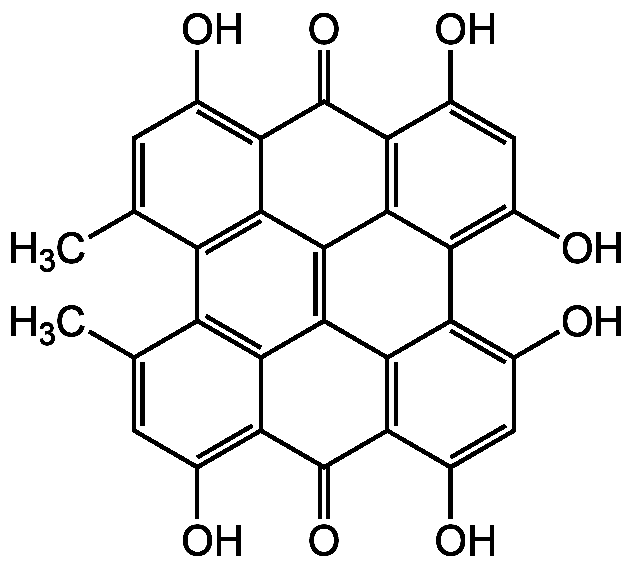
Chemical Structure
Hypericin [548-04-9]

AG-CN2-0449
Overview
- SupplierAdipoGen Life Sciences
- Product NameHypericin [548-04-9]
- Delivery Days Customer10
- CAS Number548-04-9
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC30H16O8
- Molecular Weight504.5
- Scientific DescriptionChemical. CAS: 548-04-9. Formula: C30H16O8. MW: 504.5. Isolated from Hypericum perforatum. One of the principal active constituents of St. Johns Wort (Hypericum perforatum). Hypericin is a photosensitive pigment/chromophore with bright red fluorescence emission (lambdamax: 594nm). Has a high triplet quantum yield and ability to produce considerable amounts of singlet oxygen and other ROS species when photoactivated. Antiviral, anticancer and antidepressant agent. Inactivates enveloped viruses (including HIV). This photosensitizing agent has shown to induce apoptosis and necrosis in cancer cells during photodynamic therapy. Selective inhibitor of protein kinase C (PKC). Has been shown to inhibit other kinases and targets such as EGFR-PTK, PI3K, CKII, MAPK and insulin receptor (IR). Necrosis-avid contrast agent investigated for use in non-invasively targeting necrotic tissues, based on to be defined components in the phospholipid bilayer. - One of the principal active constituents of St. Johns Wort (Hypericum perforatum). Hypericin is a photosensitive pigment/chromophore with bright red fluorescence emission (lambdamax: 594nm). Has a high triplet quantum yield and ability to produce considerable amounts of singlet oxygen and other ROS species when photoactivated. Antiviral, anticancer and antidepressant agent. Inactivates enveloped viruses (including HIV). This photosensitizing agent has shown to induce apoptosis and necrosis in cancer cells during photodynamic therapy. Selective inhibitor of protein kinase C (PKC). Has been shown to inhibit other kinases and targets such as EGFR-PTK, PI3K, CKII, MAPK and insulin receptor (IR). Necrosis-avid contrast agent investigated for use in non-invasively targeting necrotic tissues, based on to be defined components in the phospholipid bilayer.
- SMILESCC1=CC(O)=C2C(=O)C3=C4C(=C(O)C=C3O)C3=C(O)C=C(O)C5=C3C3=C4C2=C1C1=C3C(=C(O)C=C1C)C5=O
- Storage Instruction2°C to 8°C
- UNSPSC12352200
References
- On the absorption spectrum of hypericin: N. Pace & G. Mackinney; JACS 61, 3594 (1939)
- Hypericin and its photodynamic action: N. Duran & P.S. Song; Photochem. Photobiol. 43, 677 (1986) (Review)
- Hypericin and pseudohypericin specifically inhibit protein kinase C: possible relation to their antiretroviral activity: I. Takahashi, et al.; BBRC 165, 1207 (1989)
- Virucidal Activity of Hypericin Against Enveloped and Non-enveloped DNA and RNA Viruses: J. Tang, et al.; Antivir. Res. 13, 313 (1990)
- Agents for treating human immunodeficiency virus infection: E.P. Acosta & C.V. Fletcher; Am. J. Hosp. Pharm. 51, 2251 (1994) (Review)
- Photosensitized inhibition of growth factor-regulated protein kinases by hypericin: P. Agostinis, et al.; Biochem. Pharmacol. 49, 1615 (1995)
- The chemical and biological properties of hypericin - a compound with a broad spectrum of biological activities: G. Lavie, et al.; Med. Res. Rev. 15, 111 (1995) (Review)
- Natural products derived from plants as potential drugs for the photodynamic destruction of tumor cells: R. Ebermann, et al.; J. Photochem. Photobiol. B. 36, 95 (1996) (Review)
- Apoptotic and anti-apoptotic signaling pathways induced by photodynamic therapy with hypericin: P. Agostinis, et al.; Adv. Enzyme Regul. 40, 157 (2000) (Review)
- St John's wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties: J. Barnes, et al.; J. Pharm. Pharmacol. 53, 583 (2001) (Review)
