IL-2 Superkine (Fc) (H9T) [IL-2 (human):Fc (human) (rec.) (H9T)]
AG-40B-0219
Protein IDP60568
Product group Proteins / Signaling Molecules
Overview
- SupplierAdipoGen Life Sciences
- Product NameIL-2 Superkine (Fc) (H9T) [IL-2 (human):Fc (human) (rec.) (H9T)]
- Delivery Days Customer10
- CertificationResearch Use Only
- Concentration0.1 mg/ml
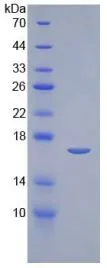
- Estimated Purity>95%
- Gene ID3558
- Target nameIL2
- Target descriptioninterleukin 2
- Target synonymsIL-2, TCGF, lymphokine, interleukin-2, T cell growth factor, aldesleukin, involved in regulation of T-cell clonal expansion
- Protein IDP60568
- Protein NameInterleukin-2
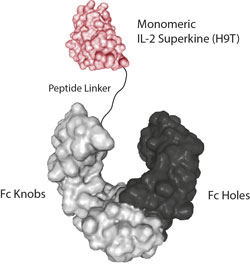
- Scientific DescriptionInterleukin-2 (IL-2) is a 133 amino acid glycoprotein with one intramolecular disulfide bond and variable glycosylation. It is secreted by activated T cells and induces proliferation and maturation of activated T cells, natural killer cells and lymphokine activated killer cells. IL-2 also stimulates proliferation of antibody-producing B cells, activates neutrophils and induces mononuclear cells to secrete IFN-gamma and TNF-alpha and -beta. Moreover, studies have shown that IL-2 is required for activation-induced apoptosis, an important homeostatic mechanism in the immune system, which is involved in the maintenance of peripheral tolerance to self-antigens. IL-2 promotes T cell proliferation and particularly naive T cells. IL-2 signaling on activated T cells is effected through a quaternary high-affinity receptor complex consisting of IL-2, IL-2Ralpha (CD25), IL-2Rbeta and IL-2Rgamma. Naive T cells are relatively insensitive to IL-2 as they only express small amounts of IL-2Rbeta and IL-2Rgamma. They only acquire sensitivity after CD25 expression, which captures the cytokine and presents it to the IL-2Rbeta and IL-2Rgamma receptors. IL-2 Superkine (Fc) is an artificial variant of IL-2 called H9, containing mutations at positions L80F / R81D / L85V / I 86V / I92F. These mutations are located in the molecules core that acts to stabilize the structure and to give it a receptor-binding conformation mimicking native IL-2 bound to CD25. These mutations effectively eliminate the functional requirement of IL-2 for CD25 expression and elicit proliferation of T cells. Compared to IL-2, the IL-2 superkine induces superior expansion of cytotoxic T cells, leading to improved anti-tumor responses in vivo, and elicits proportionally less toxicity by lowering the expansion of T-regulatory cells and reducing pulmonary oedema. A new version of IL-2 Superkine with a new additional mutation called H9T reduces the binding of IL-2Rgamma and promotes the expansion of CD8+ T cells without driving terminal differentiation. TCR-transgenic and chimeric antigen receptor-modified CD8+ T cells that are expanded with H9T showed stronger anti-tumor activity in vivo in mouse models of melanoma and acute lymphoblastic leukemia. The new variant of IL-2 called H9T, helps to maintain activated CD8+ T cells in a stem-cell-like state, with greater anti-tumor activity in two mouse models. Like IL-2 Superkine (H9), IL-2 Superkine (H9T) should also work on human T cells. - Recombinant Protein. The extracellular domain of human IL-2 (aa 21-153) (mutant H9T containing the mutations of H9: L80F / R81D / L85V / I 86V / I92F and the new mutation Q126T) is fused at the C-terminus to the Fc portion of human IgG2. Lyophilized. Contains PBS. Binds to human and mouse IL-2R. Interleukin-2 (IL-2) is a 133 amino acid glycoprotein with one intramolecular disulfide bond and variable glycosylation. It is secreted by activated T cells and induces proliferation and maturation of activated T cells, natural killer cells and lymphokine activated killer cells. IL-2 also stimulates proliferation of antibody-producing B cells, activates neutrophils and induces mononuclear cells to secrete IFN-gamma and TNF-alpha and -beta. Moreover, studies have shown that IL-2 is required for activation-induced apoptosis, an important homeostatic mechanism in the immune system, which is involved in the maintenance of peripheral tolerance to self-antigens. IL-2 promotes T cell proliferation and particularly naive T cells. IL-2 signaling on activated T cells is effected through a quaternary high-affinity receptor complex consisting of IL-2, IL-2Ralpha (CD25), IL-2Rbeta and IL-2Rgamma. Naive T cells are relatively insensitive to IL-2 as they only express small amounts of IL-2Rbeta and IL-2Rgamma. They only acquire sensitivity after CD25 expression, which captures the cytokine and presents it to the IL-2Rbeta and IL-2Rgamma receptors. IL-2 Superkine (Fc) is an artificial variant of IL-2 called H9, containing mutations at positions L80F / R81D / L85V / I 86V / I92F. These mutations are located in the molecules core that acts to stabilize the structure and to give it a receptor-binding conformation mimicking native IL-2 bound to CD25. These mutations effectively eliminate the functional requirement of IL-2 for CD25 expression and elicit proliferation of T cells. Compared to IL-2, the IL-2 superkine induces superior expansion of cytotoxic T cells, leading to improved anti-tumor responses in vivo, and elicits proportionally less toxicity by lowering the expansion of T-regulatory cells and reducing pulmonary oedema. A new version of IL-2 Superkine with a new additional mutation called H9T reduces the binding of IL-2Rgamma and promotes the expansion of CD8+ T cells without driving terminal differentiation. TCR-transgenic and chimeric antigen receptor-modified CD8+ T cells that are expanded with H9T showed stronger anti-tumor activity in vivo in mouse models of melanoma and acute lymphoblastic leukemia. The new variant of IL-2 called H9T, helps to maintain activated CD8+ T cells in a stem-cell-like state, with greater anti-tumor activity in two mouse models. Like IL-2 Superkine (H9), IL-2 Superkine (H9T) should also work on human T cells.
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116120
- SpeciesHuman



![IL-2 Superkine (Fc) [IL-2 (human):Fc (human) (rec.)]](https://adipogen.com/pub/media/catalog/product/a/g/ag-40b-0111-superkine_elisa-new.jpg)