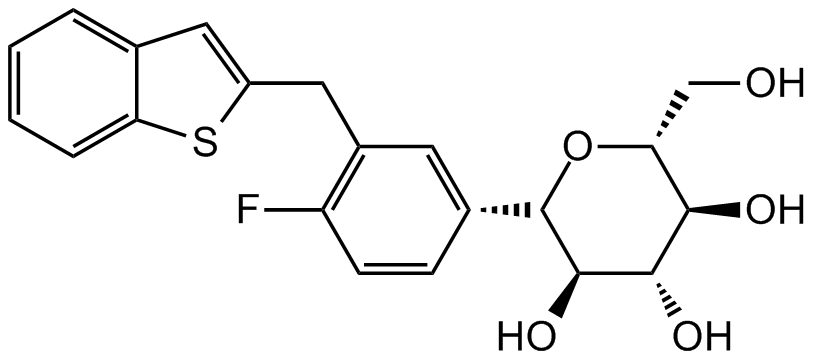
Chemical Structure
Ipragliflozin
AG-CR1-3546
CAS Number761423-87-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight404.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameIpragliflozin
- Delivery Days Customer10
- CAS Number761423-87-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC21H21FO5S
- Molecular Weight404.5
- Scientific DescriptionChemical. CAS: 761423-87-4. Formula: C21H21FO5S. MW: 404.5. Ipragliflozin is an orally active, highly potent sodium glucose co-transporter 2 (SGLT-2) inhibitor (IC50 = 7.4nM), selective over SGLT-1, 3, 4, 5 and 6. SGLT-2 is one subtype of SGLTs found almost exclusively in the proximal tubules of nephronic components in kidneys, playing a key role in the re-uptake of glucose in the proximal tubule of the kidneys. Inhibition of SGLT-2 reduces blood glucose by blocking renal glucose reabsorption and thereby increasing urinary glucose excretion (UGE). Anti-diabetic and anti-obesity agent. Shown to improve glycemic control and reduce body weight, furthermore, lowering hypoglycemic risk and abdominal symptoms. SGLT-2 inhibitors are likely to improve beta-cell function and insulin sensitivity and restore glucose homeostasis. Attenuates non-alcoholic steatohepatitis (NASH) development. - Ipragliflozin is an orally active, highly potent sodium glucose co-transporter 2 (SGLT-2) inhibitor (IC50 = 7.4nM), selective over SGLT-1, 3, 4, 5 and 6. SGLT-2 is one subtype of SGLTs found almost exclusively in the proximal tubules of nephronic components in kidneys, playing a key role in the re-uptake of glucose in the proximal tubule of the kidneys. Inhibition of SGLT-2 reduces blood glucose by blocking renal glucose reabsorption and thereby increasing urinary glucose excretion (UGE). Anti-diabetic and anti-obesity agent. Shown to improve glycemic control and reduce body weight, furthermore, lowering hypoglycemic risk and abdominal symptoms. SGLT-2 inhibitors are likely to improve beta-cell function and insulin sensitivity and restore glucose homeostasis. Attenuates non-alcoholic steatohepatitis (NASH) development.
- SMILESFC(C=CC([C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1)=C2)=C2CC3=CC4=CC=CC=C4S3
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Ipragliflozin [761423-87-4]](https://www.targetmol.com/group3/M00/02/CB/CgoaEWY7OImEOJMtAAAAAIUVYds838.png)