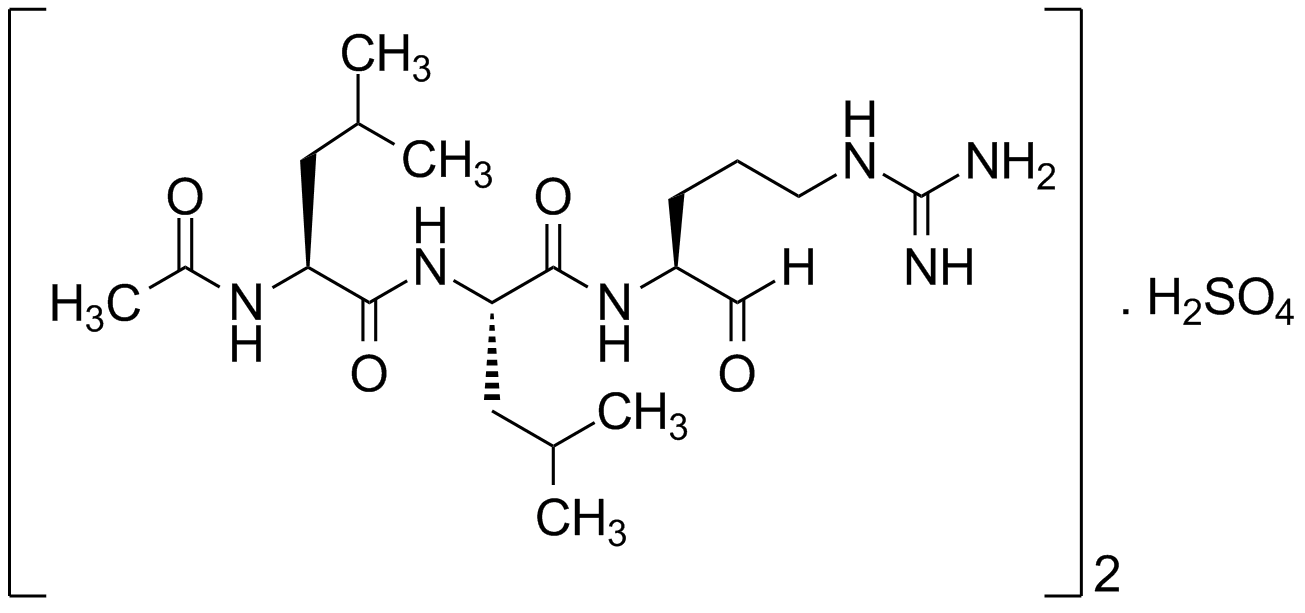
Chemical Structure
Leupeptin . hemisulfate [103476-89-7]

AG-CP3-7000
CAS Number103476-89-7
Product group Chemicals
Estimated Purity>97%
Molecular Weight426.6 . 49.0
Overview
- SupplierAdipoGen Life Sciences
- Product NameLeupeptin . hemisulfate [103476-89-7]
- Delivery Days Customer10
- CAS Number103476-89-7
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC20H38N6O4 . 0.5 H2SO4
- Molecular Weight426.6 . 49.0
- Scientific DescriptionChemical. CAS: 103476-89-7. Formula: C20H38N6O4 . 0.5 H2SO4. MW: 426.6 . 49.0. Synthetic. Potent, competitive and reversible cysteine, serine and threonine protease inhibitor. Inhibits calpain, kallikrein, trypsin, plasmin, papain and cathepsin B. Does not inhibit pepsin, elastase, renin, cathepsins A and D, thrombin or alpha-chymotrypsin. Used to protect against hearing loss caused by acoustic overstimulation or the ototoxic antibiotic gentamicin. Shown to inhibit activation-induced programmed cell death. Antioxidant and anti-inflammatory agent. Widely used as a protein purification tool to prevent proteases present in tissue samples from degrading the protein of interest. - Potent, competitive and reversible cysteine, serine and threonine protease inhibitor. Inhibits calpain, kallikrein, trypsin, plasmin, papain and cathepsin B. Does not inhibit pepsin, elastase, renin, cathepsins A and D, thrombin or alpha-chymotrypsin. Used to protect against hearing loss caused by acoustic overstimulation or the ototoxic antibiotic gentamicin. Shown to inhibit activation-induced programmed cell death. Antioxidant and anti-inflammatory agent. Widely used as a protein purification tool to prevent proteases present in tissue samples from degrading the protein of interest. Molecular docking and molecular dynamics simulation of leupeptin with the transmembrane serine protease TMPRSS2, show strong interactions with the key amino acids Ser186, His41 and Asp180 of catalytic triad present in the active site of TMPRSS2. This interaction causes the inhibition of the function of TMPRSS2, which is essential for SARS-CoV-2 viral entry and infection. As an inhibitor of TMPRSS2 this agent could be repurposed for treatment of COVID-19.
- SMILESOS(O)(=O)=O.[H]C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(C)=O.[H]C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(C)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Leupeptins, new protease inhibitors from actinomycetes: T. Aoyagi, et al.; J. Antibiot. (Tokyo) 22, 283 (1969)
- Biological activities of leupeptins: T. Aoyagi; J. Antibiot. (Tokyo) 22, 558 (1969)
- Mechanism of association of a specific aldehyde inhibitor, leupeptin, with bovine trypsin: H. Kuramochi, et al.; J. Biochem. 86, 1403 (1979)
- The slow, tight-binding inhibition of cathepsin B by leupeptin. A hysteretic effect: A. Baici, et al.; Eur. J. Biochem. 129, 33 (1982)
- Leupeptin, a thiol proteinase inhibitor, causes a selective impairment of spatial maze performance in rats: U. Staubli, et al.; Behav. Neural. Biol. 40, 58 (1984)
- Leupeptin selectively inhibits human platelet responses induced by thrombin and trypsin; a role for proteolytic activation of phospholipase C: M. Ruggiero & E.G. Lapetina; BBRC 131, 1198 (1985)
- Protease-inhibitory activities of leupeptin analogues: T. Saino, et al.; J. Antibiot. (Tokyo) 41, 220 (1988)
- New leupeptin analogues: synthesis and inhibition data: R.M. McConnell, et al.; J. Med. Chem. 33, 86 (1990)
- Developing selective inhibitors of calpain: K.K. Wang, et al.; TIPS 11, 139 (1990)
- Cell-penetrating inhibitors of calpain: S. Mehdi, et al.; TIBS 16, 150 (1991)
