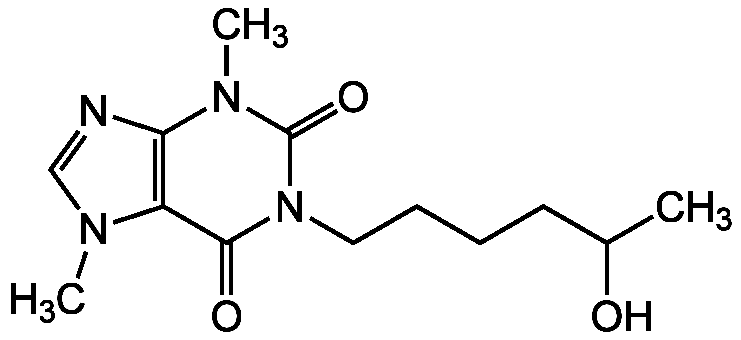
Chemical Structure
(+/-)-Lisofylline [6493-06-7] [6493-06-7]
CDX-H0117
CAS Number6493-06-7
Product group Chemicals
Estimated Purity>99%
Molecular Weight280.3
Overview
- SupplierChemodex
- Product Name(+/-)-Lisofylline [6493-06-7] [6493-06-7]
- Delivery Days Customer2
- CAS Number6493-06-7
- CertificationResearch Use Only
- Estimated Purity>99%
- Molecular FormulaC13H20N4O3
- Molecular Weight280.3
- Scientific DescriptionChemical. CAS: 6493-06-7. Formula: C13H20N4O3. MW: 280.3. Synthetic. Synthetic methylxanthine metabolite of pentoxifylline. Potent anti-inflammatory agent in which only the (-) optical isomer is biologically active. Inhibits the generation of phosphatidic acid from cytokine-activated lysophosphatidic acyl transferase (LPAAT), which has been shown to protect mice from endotoxic shock. Decreases lipid peroxidation in vitr oand in vivo. Suppresses the production of the proinflammatory cytokine IFN-gamma, inhibits IL-12-mediated STAT-4 activation, enhances glucose-stimulated beta-cell insulin secretion, reducing the onset of diabetes in a non-obese diabetic mouse model, and blocks autoimmune deterioration of pancreatic beta cells in non-obese diabetic mice. Compound can be used as analytical reference material. - Synthetic methylxanthine metabolite of pentoxifylline. Potent anti-inflammatory agent in which only the (-) optical isomer is biologically active. Inhibits the generation of phosphatidic acid from cytokine-activated lysophosphatidic acyl transferase (LPAAT), which has been shown to protect mice from endotoxic shock. Decreases lipid peroxidation in vitro and in vivo. Suppresses the production of the proinflammatory cytokine IFN-gamma, inhibits IL-12-mediated STAT-4 activation, enhances glucose-stimulated beta-cell insulin secretion, reducing the onset of diabetes in a non-obese diabetic mouse model, and blocks autoimmune deterioration of pancreatic beta cells in non-obese diabetic mice. Compound can be used as analytical reference material.
- SMILESCC(O)CCCCN1C(=O)N(C)C2=C(N(C)C=N2)C1=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![(+/-)-Lisofylline [6493-06-7]](https://www.targetmol.com/group3/M00/35/6C/CgoaEGayHvOEGcqeAAAAAG7qMcY144.png)