Chemical Structure
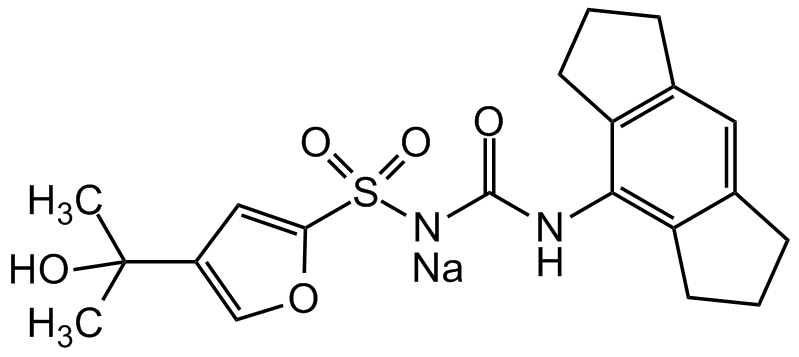
MCC950 . Na [256373-96-3]

AG-CR1-3615
Overview
- SupplierAdipoGen Life Sciences
- Product NameMCC950 . sodium salt [256373-96-3]
- Delivery Days Customer10
- CAS Number256373-96-3
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationWarning
- Molecular FormulaC20H23N2NaO5S
- Molecular Weight426.5
- Scientific DescriptionChemical. CAS: 256373-96-3. Formula: C20H23N2NaO5S. MW: 426.5. Synthetic. Potent, selective and orally available NLRP3 inflammasome inhibitor. Blocks the release of IL-1beta in macrophages primed with LPS and activated with ATP or nigericin, but it does not inhibit NLRP1, NLRC4, AIM2, TLR2 signaling or priming of NLRP3. Prevents oligomerization of ASC in cells stimulated with LPS and nigericin. Active in vivo, blocking the production of IL-1beta and enhancing survival in mouse models of multiple sclerosis and cryopyrin-associated periodic syndrome (CAPS). Also active in ex vivo samples from individuals with Muckle-Wells syndrome. Potential therapeutic agent for NLRP3-associated syndromes, including autoinflammatory and autoimmune diseases. - Potent, selective and orally available NLRP3 inflammasome inhibitor. Blocks the release of IL-1beta in macrophages primed with LPS and activated with ATP or nigericin, but it does not inhibit NLRP1, NLRC4, AIM2, TLR2 signaling or priming of NLRP3. Prevents oligomerization of ASC in cells stimulated with LPS and nigericin. Active in vivo, blocking the production of IL-1beta and enhancing survival in mouse models of multiple sclerosis and cryopyrin-associated periodic syndrome (CAPS). Also active in ex vivo samples from individuals with Muckle-Wells syndrome. Potential therapeutic agent for NLRP3-associated syndromes, including autoinflammatory and autoimmune diseases.
- SMILES[NaH].CC(C)(O)C1=COC(=C1)S(=O)(=O)NC(=O)NC1=C2CCCC2=CC2=C1CCC2
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Glutathione s-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1beta posttranslational processing: R.E. Laliberte, et al.; J. Biol. Chem. 278, 16567 (2003)
- Novel synthesis of 1-(1,2,3,5,6,7-hexahydro-s-indacen-4-yl)-3-[4-(1-hydroxy-1-methylethyl)furan-2-sulfonyl]urea, an antiinflammatory agent: F.J. Urban, et al.; Synth. Commun. 33, 2029 (2003)
- The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes: R.C. Coll, et al.; PLoS One 6, e29539 (2011)
- A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases: R.C. Coll, et al.; Nat. Med. 21, 248 (2015)
- Taming the inflammasome: M. Levy, et al.; Nat. Med. 21, 213 (2015)
- The Nlrp3 inflammasome admits defeat: C.J. Gross & O. Gross; Trends Immunol. 36, 323 (2015)
