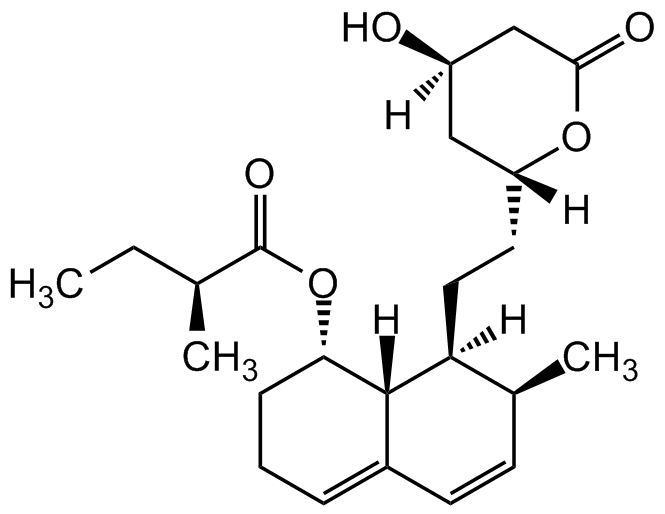
Chemical Structure
Mevastatin [73573-88-3] [73573-88-3]
CDX-M0183
CAS Number73573-88-3
Product group Chemicals
Estimated Purity>95%
Molecular Weight390.51
Overview
- SupplierChemodex
- Product NameMevastatin [73573-88-3] [73573-88-3]
- Delivery Days Customer10
- CAS Number73573-88-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC23H34O5
- Molecular Weight390.51
- Scientific DescriptionChemical. CAS: 73573-88-3. Formula: C23H34O5. MW: 390.51. Mevastatin is an antibiotic that is a competitive HMG-CoA reductase inhibitor, thus suppressing Ras farnesylation. Mevastatin decreases cholesterol biosynthesis in vitro and in vivo. It induces apoptosis, arrests cancer cells in G1 phase and downregulates cdk 2, 4, and 6, cyclin D1 and E1, p21 and p27. Mevastatin also suppresses TNF-induced NF-kappaB activation and potentiates apoptosis in human myeloid leukemia cells. Mevastatin induced neurite outgrowth of neuroblastoma cells via activation of EGFR, ERK1/2 and Akt/PBK but also exerts a neurotoxic effect in cultured neurons in a heme-independent manner. Mevastatin also shows antileishmanial and antifungal activity. Has also anti-inflammatory effects potentially via up-regulation of heme oxygenase-1 (HO-1). - Mevastatin is an antibiotic that is a competitive HMG-CoA reductase inhibitor, thus suppressing Ras farnesylation. Mevastatin decreases cholesterol biosynthesis in vitro and in vivo. It induces apoptosis, arrests cancer cells in G1 phase and downregulates cdk 2, 4, and 6, cyclin D1 and E1, p21 and p27. Mevastatin also suppresses TNF-induced NF-kappaB activation and potentiates apoptosis in human myeloid leukemia cells. Mevastatin induced neurite outgrowth of neuroblastoma cells via activation of EGFR, ERK1/2 and Akt/PBK but also exerts a neurotoxic effect in cultured neurons in a heme-independent manner. Mevastatin also shows antileishmanial and antifungal activity. Has also anti-inflammatory effects potentially via up-regulation of heme oxygenase-1 (HO-1).
- SMILES[H][C@@]12C(C=C[C@H](C)[C@]2([H])CC[C@@]3([H])OC(C[C@@](O)([H])C3)=O)=CCC[C@@H]1OC([C@@H](C)CC)=O
- Storage Instruction2°C to 8°C
- UNSPSC12352200


![Mevastatin [73573-88-3] [73573-88-3]](https://www.targetmol.com/group3/M00/02/14/CgoaEGY7KB2EDfONAAAAAPY9yhQ262.png)