MMP9 Antibody: RPE
ORB149764
ApplicationsImmunoFluorescence, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry
Product group Antibodies
ReactivityHuman, Mouse, Rat
TargetMmp9
Overview
- SupplierBiorbyt
- Product NameMMP9 Antibody: RPE
- Delivery Days Customer16
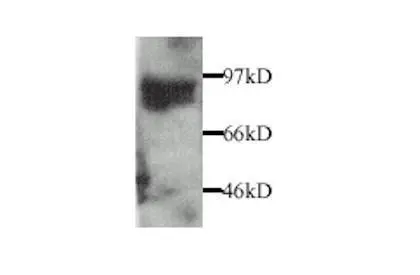
- Application Supplier Note1 microg/ml of SMC-396 was sufficient for detection of MMP9 in 20 microg of COS-1 cells (lysate) transfected with human MMP9 by colorimetric immunoblot analysis using goat anti-mouse IgG:HRP as the secondary antibody.
- ApplicationsImmunoFluorescence, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry
- Applications SupplierWB (1:1000), ICC/IF (1:100) ICC, IF, IHC, WB
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDL51/82 (Formerly sold as S51-82)
- Concentration1 mg/ml
- ConjugateRPE
- Gene ID81687
- Target nameMmp9
- Target descriptionmatrix metallopeptidase 9
- Target synonymsmatrix metalloproteinase-9, 92 kDa gelatinase, 92-kDa type IV collagenase, GELB, MMP-9, gelatinase B, matrix metalloproteinase 9 (gelatinase B, 92-kDa type IV collagenase)
- HostMouse
- IsotypeIgG2a
- Protein IDP50282
- Protein NameMatrix metalloproteinase-9
- Scientific DescriptionMouse monoclonal to MMP9 (RPE). MMP9, otherwise known as matrix metalloproteinase 9, is involved in the breakdown of extracellular matrix in normal physiological processes such as embryonic development, reproduction and tissue remodeling, as well as in disease processes like arthritis and metastasis. Among the family members, MMP-2, MMP-3, MMP-7 and MMP-9 have been characterized as important factors for normal tissue remodeling during embryonic development, wound healing, tumor invasion, angiogenesis, carcinogenesis and apoptosis (2-4). MMP activity correlates with cancer development. One mechanism of MMP regulation is transcriptional. Once synthesized, MMP exists as a latent proenzyme. Maximum MMP activity requires proteolytic cleavage to generate active MMPs by releasing the inhibitory propeptide domain from the full length protein..
- ReactivityHuman, Mouse, Rat
- Storage InstructionSee Manual
- UNSPSC12352203