Chemical Structure
N1-Guanyl-1,7-diaminoheptane [GC7]

AG-CR1-3702
Overview
- SupplierAdipoGen Life Sciences
- Product NameN1-Guanyl-1,7-diaminoheptane [GC7] [150333-69-0, 150417-90-6]
- Delivery Days Customer10
- CAS Number150333-69-0
- CertificationResearch Use Only
- Estimated Purity>98%
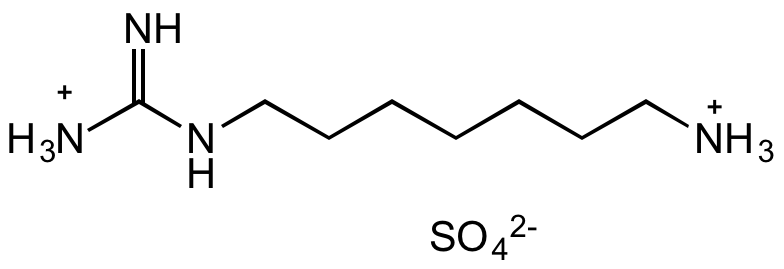
- Molecular FormulaC8H22N4O4S
- Molecular Weight270
- Scientific DescriptionCell permeable competitive deoxyhypusine synthase (DHPS) inhibitor. Targets the spermidine-binding site of deoxyhypusine synthase (Ki = 9.7 nM), and prevents the first step in the post-translational conversion of a single lysine to hypusine on eukaryotic initiation factor 5A (eIF5A). The modification is essential for sustained cell growth and therefore eIF5A hypusination controls cell proliferation and has been linked to cancer. Hypusination of eIF-5aA in macrophages is required for the TCA/OXPHOS pathways by regulating the expression of the important enzymes of the pathways. GC7 blocks the expression of the key enzymes (at the level of translation) and blocks OXPHOS in macrophages. Useful tool for immunometabolism research. The polyamine spermidine as a substrate to generate hypusinated eIF5A. Elevated levels of polyamines are a hallmark of most tumor types. Agents that block the function of key biosynthetic enzymes in the polyamine pathway markedly impair tumor progression and maintenance of the malignant state. Anti-tumor agent in vivo. Anti-proliferative agent in several solid tumors. Shown to increase the chemosensitivity to other anticancer drugs. Inhibits HIF-1alpha expression and activates autophagy independent of DHPS. Inhibition of deoxyhypusine synthase may provide a strategy for reducing diabetogenic Th1 cells and preserving beta cell function in type 1 diabetes. Hypusinated eIF5A has been demonstrated to have a proinflammatory role in the release of cytokines and the production of NO and might be an interesting cellular target for anti-inflammatory treatments. - Chemical. CAS: 150417-90-6 (parent 150333-69-0). Formula: C8H22N4O4S. MW: 270. Cell permeable competitive deoxyhypusine synthase (DHPS) inhibitor. Targets the spermidine-binding site of deoxyhypusine synthase (Ki = 9.7 nM), and prevents the first step in the post-translational conversion of a single lysine to hypusine on eukaryotic initiation factor 5A (eIF5A). The modification is essential for sustained cell growth and therefore eIF5A hypusination controls cell proliferation and has been linked to cancer. Hypusination of eIF-5aA in macrophages is required for the TCA/OXPHOS pathways by regulating the expression of the important enzymes of the pathways. GC7 blocks the expression of the key enzymes (at the level of translation) and blocks OXPHOS in macrophages. Useful tool for immunometabolism research. The polyamine spermidine as a substrate to generate hypusinated eIF5A. Elevated levels of polyamines are a hallmark of most tumor types. Agents that block the function of key biosynthetic enzymes in the polyamine pathway markedly impair tumor progression and maintenance of the malignant state. Anti-tumor agent in vivo. Anti-proliferative agent in several solid tumors. Shown to increase the chemosensitivity to other anticancer drugs. Inhibits HIF-1alpha expression and activates autophagy independent of DHPS. Inhibition of deoxyhypusine synthase may provide a strategy for reducing diabetogenic Th1 cells and preserving beta cell function in type 1 diabetes. Hypusinated eIF5A has been demonstrated to have a proinflammatory role in the release of cytokines and the production of NO and might be an interesting cellular target for anti-inflammatory treatments.
- SMILES[NH3+]C(NCCCCCCC[NH3+])=N.O=S([O-])([O-])=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
