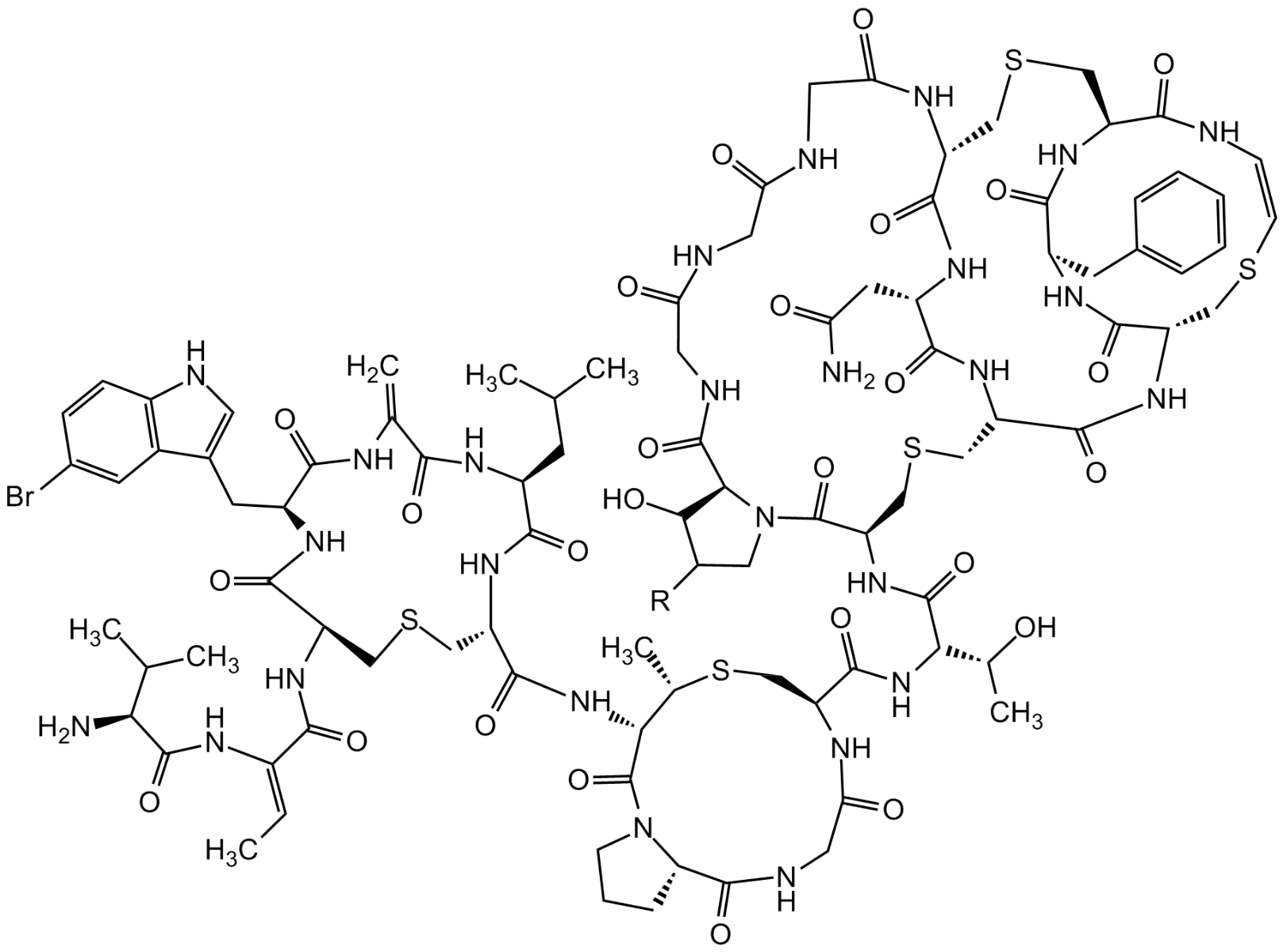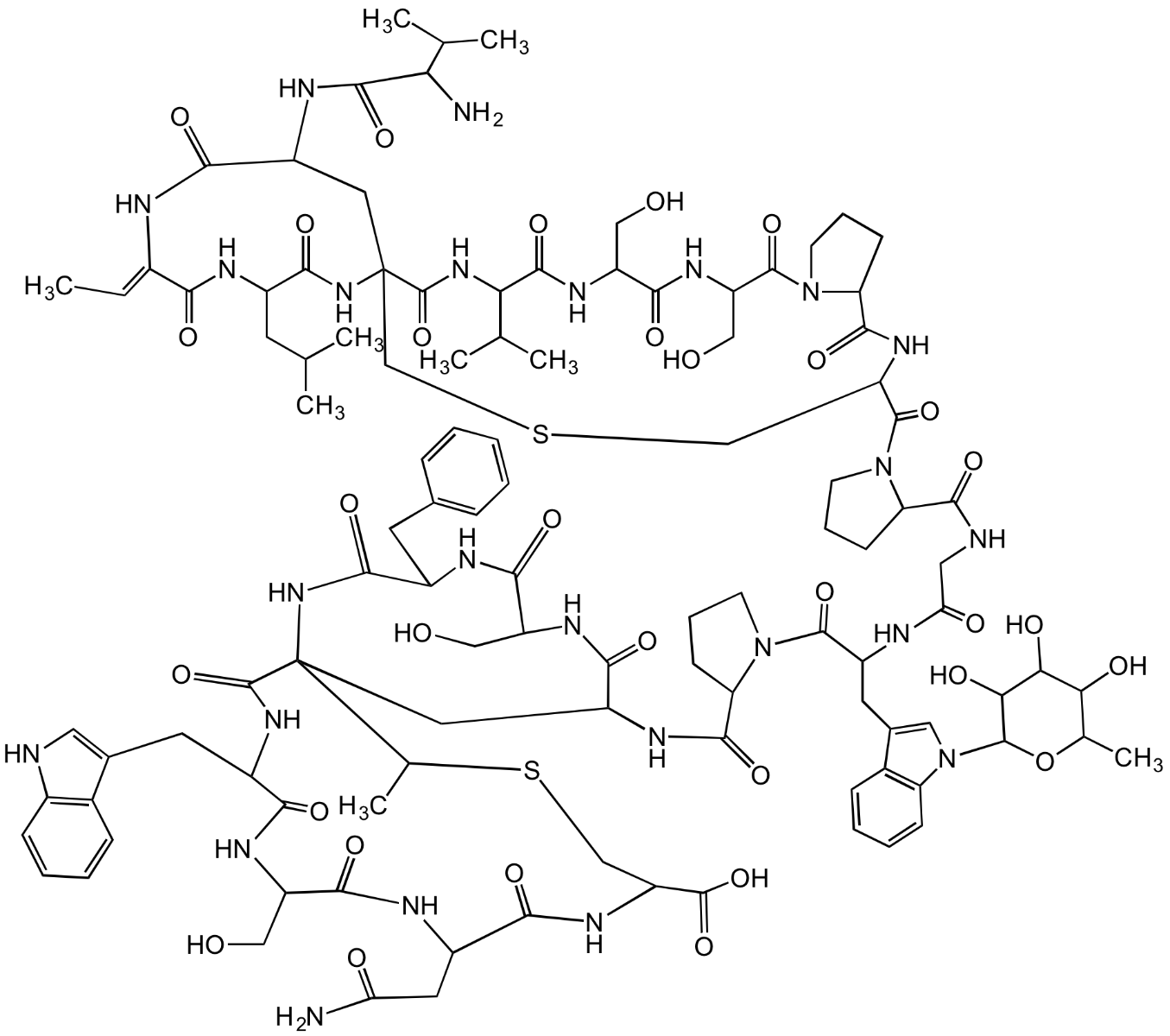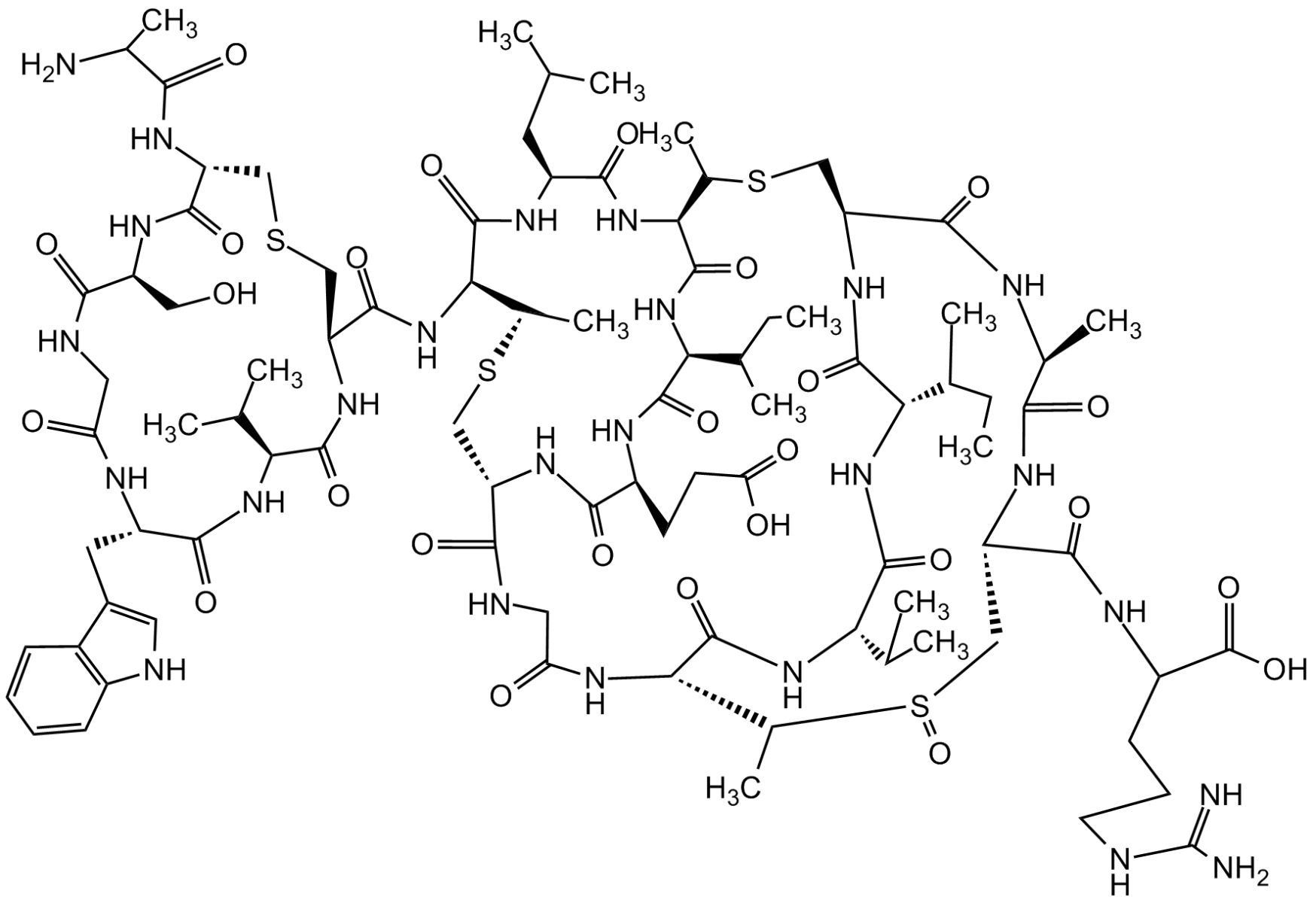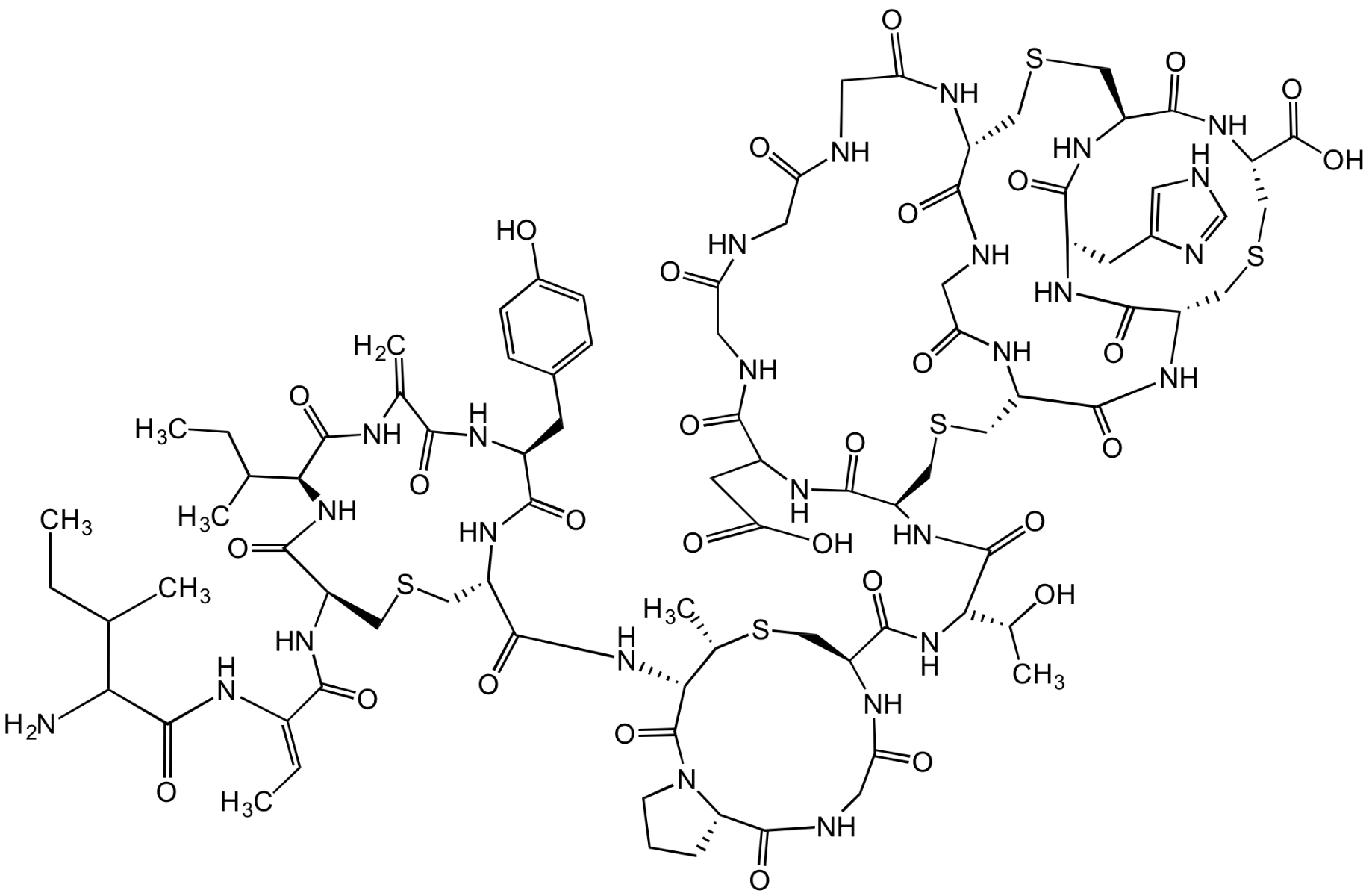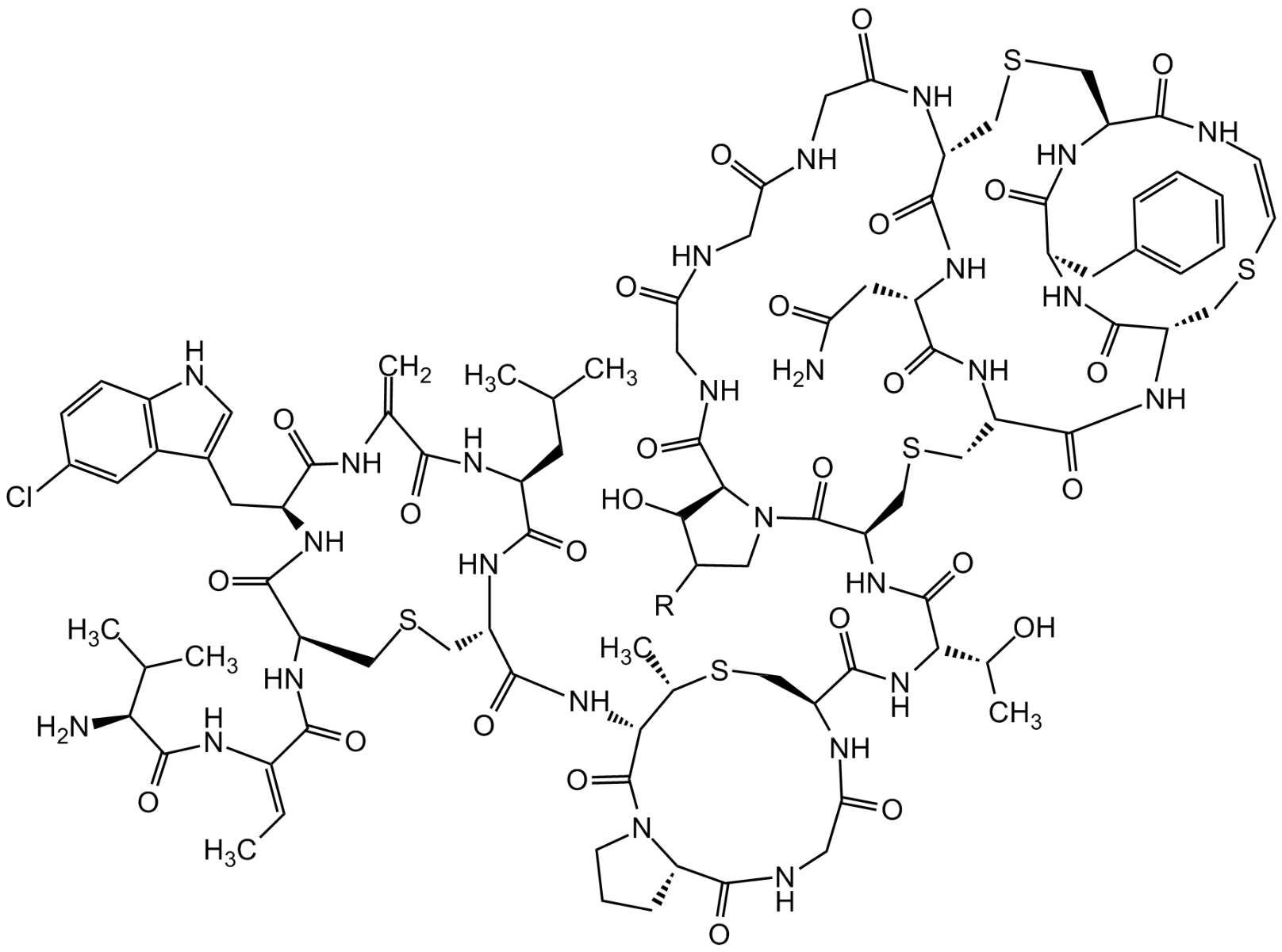
Chemical Structure
NAI-107 [Microbisporicin] [845293-74-5]
AG-CN2-0307
CAS Number845293-74-5
Product group Chemicals
Estimated Purity>80%
Molecular Weight2249.0 (A1; R=OH)2233.0 (A2; R=H)
Overview
- SupplierAdipoGen Life Sciences
- Product NameNAI-107 [Microbisporicin] [845293-74-5]
- Delivery Days Customer10
- CAS Number845293-74-5
- CertificationResearch Use Only
- Estimated Purity>80%
- Molecular FormulaC94H127ClN26O27S5 (A1) C94H127ClN26O26S5 (A2)
- Molecular Weight2249.0 (A1; R=OH)2233.0 (A2; R=H)
- Scientific DescriptionAntibacterial class I lantibiotic. Mixture of two similarly active and structurally related polypeptides (A1, 2246Da and A2, 2230Da) of 24 amino acids linked by 5 intramolecular thioether bridges. Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis. Efficiently interferes with late stages of cell wall biosynthesis leading to accumulation of the soluble peptidoglycan precursor UDP-N-acetylmuramic acid-pentapeptide (UDP-MurNAc-pentapeptide) in the cytoplasm. Active against aerobic and anaerobic Gram-positive pathogens, including all antibiotic-resistant strains (e.g. MRSA and VRE) in whole cell and in vitro assays as well as in vivo. Rapidly bactericidal and highly efficacious in experimental models of infection (septicemia, endocarditis, granuloma pouch) and developed for treatment of serious infections by multidrug-resistant Gram-positive bacteria. - Chemical. CAS: 845293-74-5 [A1/A2 Mixture]. Formula: C94H127ClN26O27S5 (A1), C94H127ClN26O26S5 (A2). MW: 2249.0 (A1; R=OH)2233.0 (A2; R=H). Isolated from Microbispora sp. Antibacterial class I lantibiotic. Mixture of two similarly active and structurally related polypeptides (A1, 2246Da and A2, 2230Da) of 24 amino acids linked by 5 intramolecular thioether bridges. Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis. Efficiently interfers with late stages of cell wall biosynthesis leading to accumulation of the soluble peptidoglycan precursor UDP-N-acetylmuramic acid-pentapeptide (UDP-MurNAc-pentapeptide) in the cytoplasm. Active against aerobic and anaerobic Gram-positive pathogens, including all antibiotic-resistant strains (e.g. MRSA and VRE) in whole cell and in vitro assays as well as in vivo. Rapidly bactericidal and highly efficacious in experimental models of infection (septicemia, endocarditis, granuloma pouch) and developed for treatment of serious infections by multiresistant Gram-positive bacteria.
- SMILESClC1=CC=C2C(C(C[C@@H]3NC([C@@H](CSC[C@H](NC([C@H](CC(C)C)NC(C(NC3=O)=C)=O)=O)C(N[C@@H]4C(N5[C@@](C(NCC(N[C@H](C(N[C@]([C@H](O)C)([H])C(N[C@H]6C(N7[C@@](C(NCC(NCC(NCC(N[C@@]8([H])C(N[C@@H](CC(N)=O)C(N[C@@](C(N[C@@]9([H])C(N[C@H](C(N[C@](CSC8)([H])C(N/C=C\SC9)=O)=O)CC%10=CC=CC=C%10)=O)=O)([H])CSC6)=O)=O)=O)=O)=O)=O)([H])C([H])C(O)C7)=O)=O)=O)CS[C@H]4C)=O)=O)([H])CCC5)=O)=O)NC(/C(NC([C@@H](N)C(C)C)=O)=C/C)=O)=O)=CN2)=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200