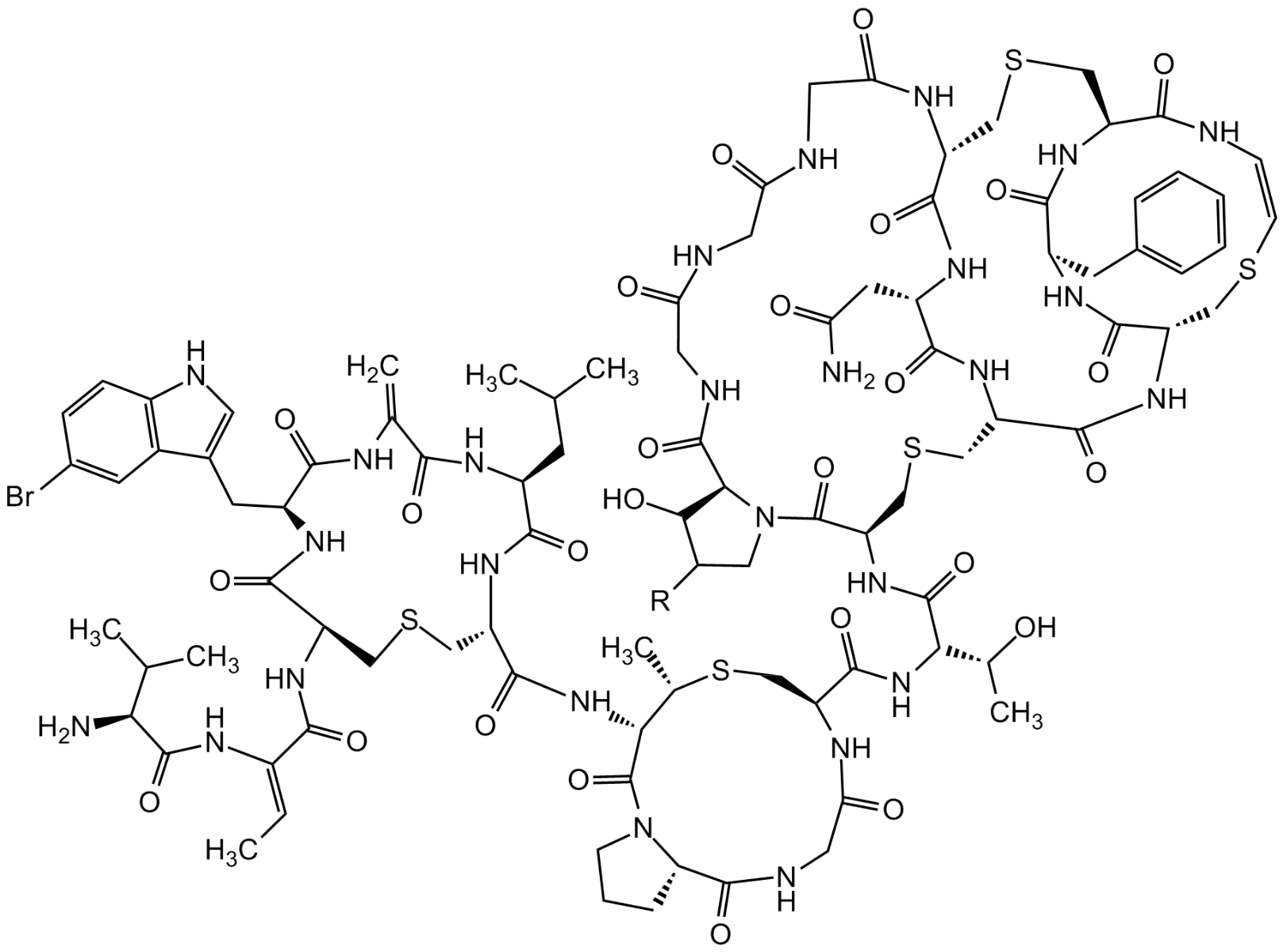
Chemical Structure
NAI-108 [1820892-86-1]

AG-CN2-0308
CAS Number1820892-86-1
Product group Chemicals
Estimated Purity>80%
Molecular Weight2293.4 (A1; R=OH)2277.4 (A2; R=H)
Overview
- SupplierAdipoGen Life Sciences
- Product NameNAI-108 [1820892-86-1]
- Delivery Days Customer10
- CAS Number1820892-86-1
- CertificationResearch Use Only
- Estimated Purity>80%
- Molecular FormulaC94H127BrN26O27S5 (A1) C94H127BrN26O26S5 (A2)
- Molecular Weight2293.4 (A1; R=OH)2277.4 (A2; R=H)
- Scientific DescriptionAntibacterial class I lantibiotic. Brominated variant of NAI-107 (AG-CN2-0307 https://adipogen.com/ag-cn2-0307-nai-107-microbisporicin.html ). Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis. Efficiently interfers with late stages of cell wall biosynthesis leading to accumulation of the soluble peptidoglycan precursor UDP-N-acetylmuramic acid-pentapeptide (UDP-MurNAc-pentapeptide) in the cytoplasm. Slightly higher activity than NAI-107 against multidrug-resistant Gram-positive pathogens. - Chemical. CAS: 1820892-86-1. Formula: C94H127BrN26O27S5 (A1), C94H127BrN26O26S5 (A2). MW: 2293.4 (A1; R=OH)2277.4 (A2; R=H). Isolated from Actinoallomurus sp. Antibacterial class I lantibiotic. Brominated variant of NAI-107. Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis. Efficiently interfers with late stages of cell wall biosynthesis leading to accumulation of the soluble peptidoglycan precursor UDP-N-acetylmuramic acid-pentapeptide (UDP-MurNAc-pentapeptide) in the cytoplasm. Slightly higher activity than NAI-107 against multidrug-resistant Gram-positive pathogens.
- SMILESO=C(N[C@H](C(NC(C(N[C@H](C(N[C@H](C(N[C@H]([C@H](C)SC[C@@H](C(N[C@H]([C@H](O)C)C(N[C@@H](C(N1[C@H]2[C@@H](O)CC1)=O)CSC[C@H](NC([C@H](CC(N)=O)NC([C@H](NC(CNC(CNC(CNC2=O)=O)=O)=O)CSC[C@@H](C(N/C=C\SC3)=O)NC4=O)=O)=O)C(N[C@@H]3C(N[C@@H]4CC5=CC=CC=C5)=O)=O)=O)=O)NC6=O)C(N7[C@H](C(NC6)=O)CCC7)=O)=O)CSC8)=O)CC(C)C)=O)=C)=O)CC9=CNC%10=C9C=C(Br)C=C%10)[C@@H]8NC(/C(NC([C@H](C(C)C)N)=O)=C/C)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Brominated Variant of the Lantibiotic NAI-107 with Enhanced Antibacterial Potency: J.C. Cruz, et al.; J. Nat. Prod. 78, 2642 (2015)
