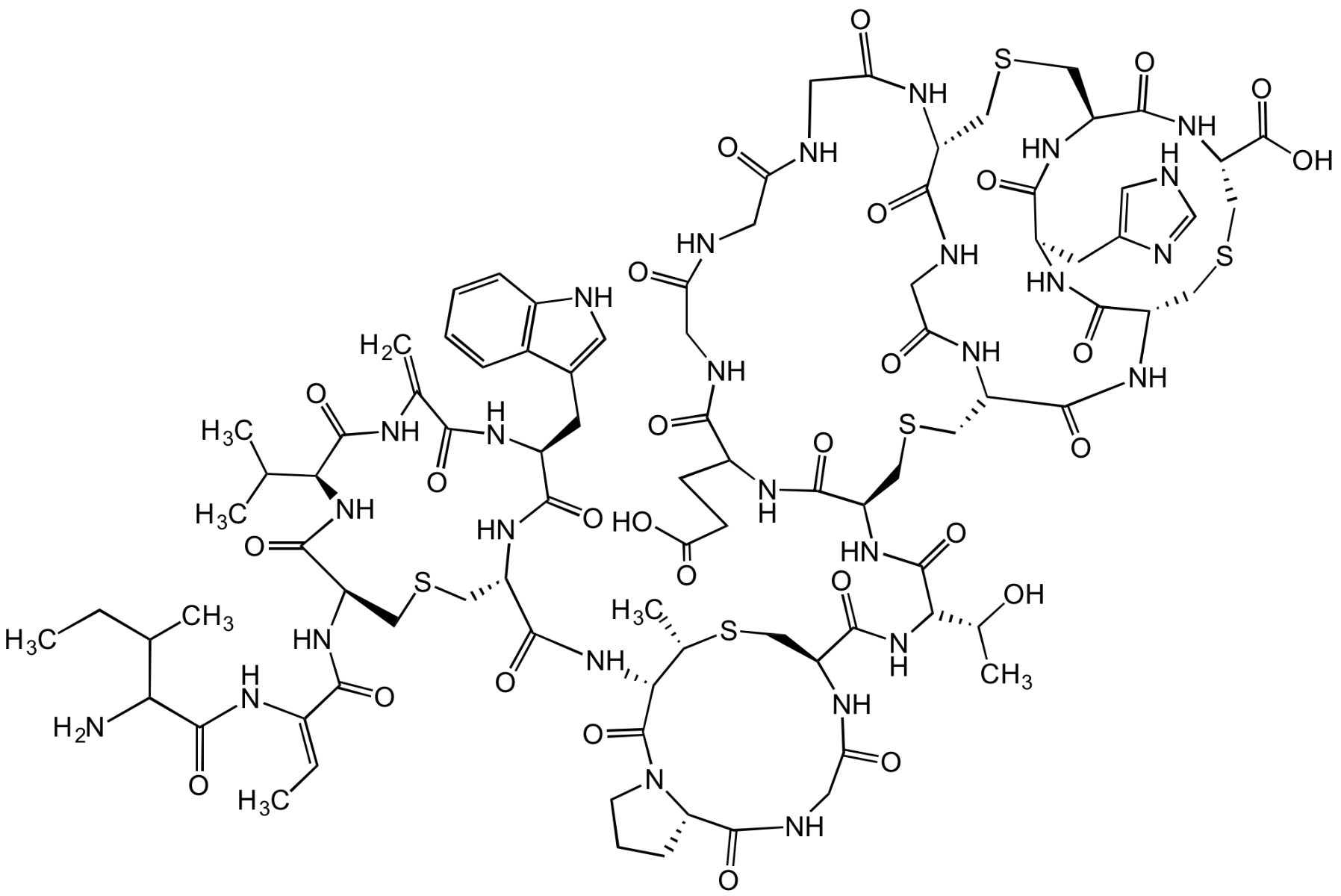
Chemical Structure
NAI-97 [Planosporicin]

AG-CN2-0312
Overview
- SupplierAdipoGen Life Sciences
- Product NameNAI-97 [Planosporicin] [802917-82-4]
- Delivery Days Customer10
- CAS Number802917-82-4
- CertificationResearch Use Only
- Estimated Purity>75%
- Molecular FormulaC90H125N27O28S5
- Molecular Weight2193.5
- Scientific DescriptionAntibacterial class I lantibiotic. Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis. Interferes with late stages of cell wall biosynthesis leading to accumulation of the soluble peptidoglycan precursor UDP-N-acetylmuramic acid-pentapeptide (UDP-MurNAc-pentapeptide) in the cytoplasm less efficient than NAI-107 (AG-CN2-0307 https://adipogen.com/ag-cn2-0307-nai-107-microbisporicin.html ). Active against aerobic and anaerobic Gram-positive pathogens, including all antibiotic-resistant strains (e.g. MRSA and VRE) in whole cell and in vitro assays as well as in vivo. - Chemical. CAS: 802917-82-4. Formula: C90H125N27O28S5. MW: 2193.5. Isolated from Planomonospora sp. Antibacterial class I lantibiotic. Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis. Interfers with late stages of cell wall biosynthesis leading to accumulation of the soluble peptidoglycan precursor UDP-N-acetylmuramic acid-pentapeptide (UDP-MurNAc-pentapeptide) in the cytoplasm less efficient than NAI-107. Active against aerobic and anaerobic Gram-positive pathogens, including all antibiotic-resistant strains (e.g. MRSA and VRE) in whole cell and in vitro assays as well as in vivo.
- SMILESO=C(N[C@H](C(NC(C(N[C@H]1CC2=CNC3=C2C=CC=C3)=O)=C)=O)C(C)C)[C@H](NC(/C(NC(C(N)C(C)CC)=O)=C/C)=O)CSC[C@@H](C(N[C@@H](C(N4[C@H](C(NCC(N5)=O)=O)CCC4)=O)[C@H](C)SC[C@H]5C(N[C@H]([C@H](O)C)C(N[C@@H](C(NC(CCC(O)=O)C(NCC(NCC(NCC(N6)=O)=O)=O)=O)=O)CSC[C@@H](C(N[C@@H](CSC7)C(N[C@@H]8CC9=CNC=N9)=O)=O)NC(CNC([C@H]6CSC[C@@H](C(N[C@@H]7C(O)=O)=O)NC8=O)=O)=O)=O)=O)=O)NC1=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- A novel lantibiotic acting on bacterial cell wall synthesis produced by the uncommon actinomycete Planomonospora sp: F. Castiglione, et al.; Biochemistry 46, 5884 (2007)
- Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens: F. Castiglione, et al.; Chem. Biol. 15, 22 (2008)
- Structure revision of the lantibiotic 97518: S.I. Maffioli, et al.; J. Nat. Prod. 72, 605 (2009)
- Advancing cell wall inhibitors towards clinical applications: S.I. Maffioli, et al.; J. Ind. Microbiol. Biotechnol. 43, 177 (2016) (Review)
