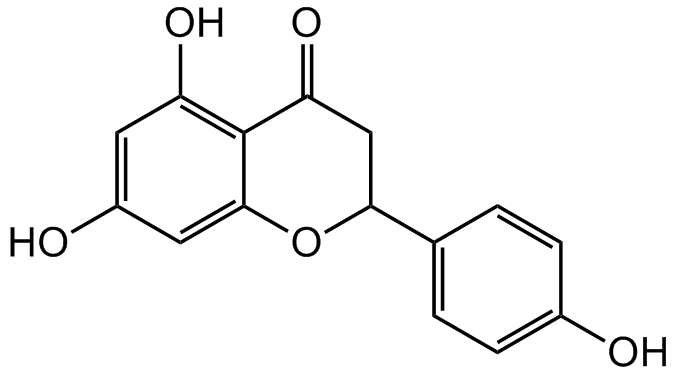
Chemical Structure
(+/-)-Naringenin [67604-48-2] [67604-48-2]
CDX-N0206
CAS Number67604-48-2
Product group Chemicals
Estimated Purity>98%
Molecular Weight272.25
Overview
- SupplierChemodex
- Product Name(+/-)-Naringenin [67604-48-2] [67604-48-2]
- Delivery Days Customer2
- CAS Number67604-48-2
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC15H12O5
- Molecular Weight272.25
- Scientific DescriptionChemical. CAS: 67604-48-2. Formula: C15H12O5. MW: 272.25. Naringenin is a citrus-derived flavonoid that inhibits CYP3A4 activity in human liver microsomes. It has a broad panel of properties, including antibacterial, antiviral, antifungal, anti-inflammatory, antioxidant, anticancer, antidiabetic, cardioprotective and neuroprotective activities. Naringenin activity is primarily attributed to its anti-inflammatory (via inhibiting recruitment of cytokines and inflammatory transcription factors) and antioxidant (via scavenging of free radicals, bolstering of endogenous antioxidant defense system and metal ion chelation) effects. Naringenin exerts its anti-diabetic effects by inhibition of gluconeogenesis through upregulations of AMPK, leading to its lipid lowering and insulin-like properties. - Naringenin is a citrus-derived flavonoid that inhibits CYP3A4 activity in human liver microsomes. It has a broad panel of properties, including antibacterial, antiviral, antifungal, anti-inflammatory, antioxidant, anticancer, antidiabetic, cardioprotective and neuroprotective activities. Naringenin activity is primarily attributed to its anti-inflammatory (via inhibiting recruitment of cytokines and inflammatory transcription factors) and antioxidant (via scavenging of free radicals, bolstering of endogenous antioxidant defense system and metal ion chelation) effects. Naringenin exerts its anti-diabetic effects by inhibition of gluconeogenesis through upregulations of AMPK, leading to its lipid lowering and insulin-like properties.
- SMILESOC1=CC(O)=C2C(OC(C3=CC=C(O)C=C3)CC2=O)=C1
- Storage Instruction2°C to 8°C
- UNSPSC12352200


![(+/-)-Naringenin [67604-48-2]](https://www.targetmol.com/group3/M00/03/59/CgoaEWY7SNWEJoilAAAAANLm8iQ549.png)