Chemical Structure
Nexturastat B [1648893-33-7]

AG-CR1-3902
Overview
- SupplierAdipoGen Life Sciences
- Product NameNexturastat B [1648893-33-7]
- Delivery Days Customer10
- CAS Number1648893-33-7
- CertificationResearch Use Only
- Estimated Purity>98%
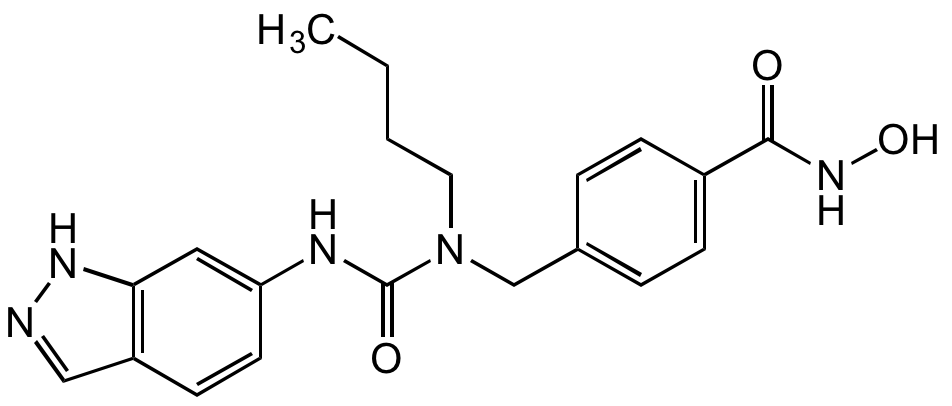
- Molecular FormulaC20H23N5O3
- Molecular Weight381.4
- Scientific DescriptionCell permeable, potent and selective class IIb HDAC6 inhibitor (IC50=3nM). Displays high selectivity over HDAC1 (300-fold). More soluble analog of nexturastat A (Prod. No. AG-CR1-3901). Suppresses cell proliferation of B16 cells (GI50=14.3microM) and human lymphoma cells HuT-78 at a slightly lower concentration than nexturastat A. Shown to be effective in a mouse melanoma tumor xenograft model, increasing tumor volumes in vivo. Down-regulates the production of IL-10. HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration. - Chemical. CAS: 1648893-33-7. Formula: C20H23N5O3. MW: 381.4. Cell permeable, potent and selective class IIb HDAC6 inhibitor (IC50=3nM). Displays high selectivity over HDAC1 (300-fold). More soluble analog of nexturastat A (Prod. No. AG-CR1-3901). Suppresses cell proliferation of B16 cells (GI50=14.3microM) and human lymphoma cells HuT-78 at a slightly lower concentration than nexturastat A. Shown to be effective in a mouse melanoma tumor xenograft model, increasing tumor volumes in vivo. Down-regulates the production of IL-10. HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration.
- SMILESCCCCN(CC1=CC=C(C=C1)C(=O)NO)C(=O)NC1=CC2=C(C=NN2)C=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Targeting histone deacetylase 6 mediates a dual anti-melanoma effect: Enhanced antitumor immunity and impaired cell proliferation: K.V. Woan, et al.; Mol. Oncol. 9, 1447 (2015)
- Exploration of some new HDAC inhibitors for cancer and CNS diseases: A.P. Kozikowski; 13th Annual Discovery on Target (presentation) (2015)
