Chemical Structure
ODQ [41443-28-1]

AG-CR1-3500
Overview
- SupplierAdipoGen Life Sciences
- Product NameODQ [41443-28-1]
- Delivery Days Customer10
- CAS Number41443-28-1
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC9H5N3O2
- Molecular Weight187.2
- Scientific DescriptionChemical. CAS: 41443-28-1. Formula: C9H5N3O2. MW: 187.2. Potent and highly selective, irreversible inhibitor of soluble guanylyl cyclase (sGC). The binding is competitive with nitric oxide (NO). Tool to elucidate the nitric oxide (NO)-cGMP pathway. Apoptosis inhibitor Inhibits growth and migration of prostate cancer cells independent of its effects on GMP levels. - Potent and highly selective, irreversible inhibitor of soluble guanylyl cyclase (sGC). The binding is competitive with nitric oxide (NO) [1-5]. Tool to elucidate the nitric oxide (NO)-cGMP pathway [1-5]. Apoptosis inhibitor [6] Inhibits growth and migration of prostate cancer cells independent of its effects on GMP levels [6].
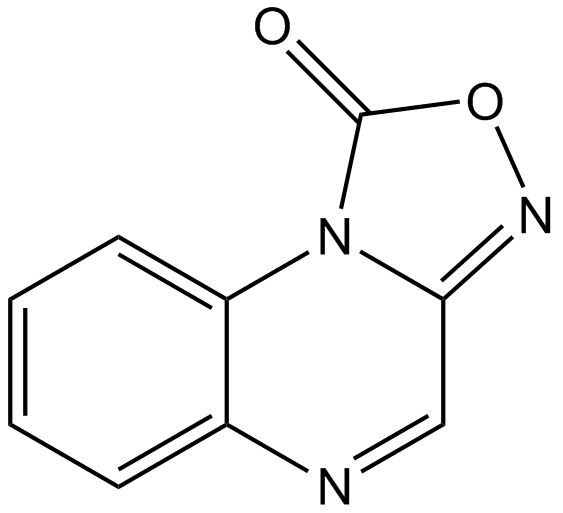
- SMILESO=C1ON=C2C=NC3=CC=CC=C3N12
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Nitric oxide-dependent long-term potentiation is blocked by a specific inhibitor of soluble guanylyl cyclase: C.L. Boulton, et al.; Neuroscience 69, 699 (1995)
- Novel guanylyl cyclase inhibitor, ODQ reveals role of nitric oxide, but not of cyclic GMP in endothelin-1 secretion: F. Brunner, et al.; FEBS Lett. 376, 262 (1995)
- Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one: J. Garthwaite, et al.; Mol. Pharmacol. 48, 184 (1995)
- Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase: A. Schrammel, et al.; Mol. Pharmacol. 50, 1 (1996)
- Inhibition of Soluble Guanylate Cyclase by ODQ: Y. Zhao, et al.; Biochemistry 39, 10848 (2000)
- cGMP-independent anti-tumour actions of the inhibitor of soluble guanylyl cyclase, ODQ, in prostate cancer cell lines: G. Haramis, et al.; Br. J. Pharmacol. 155, 804 (2008)
