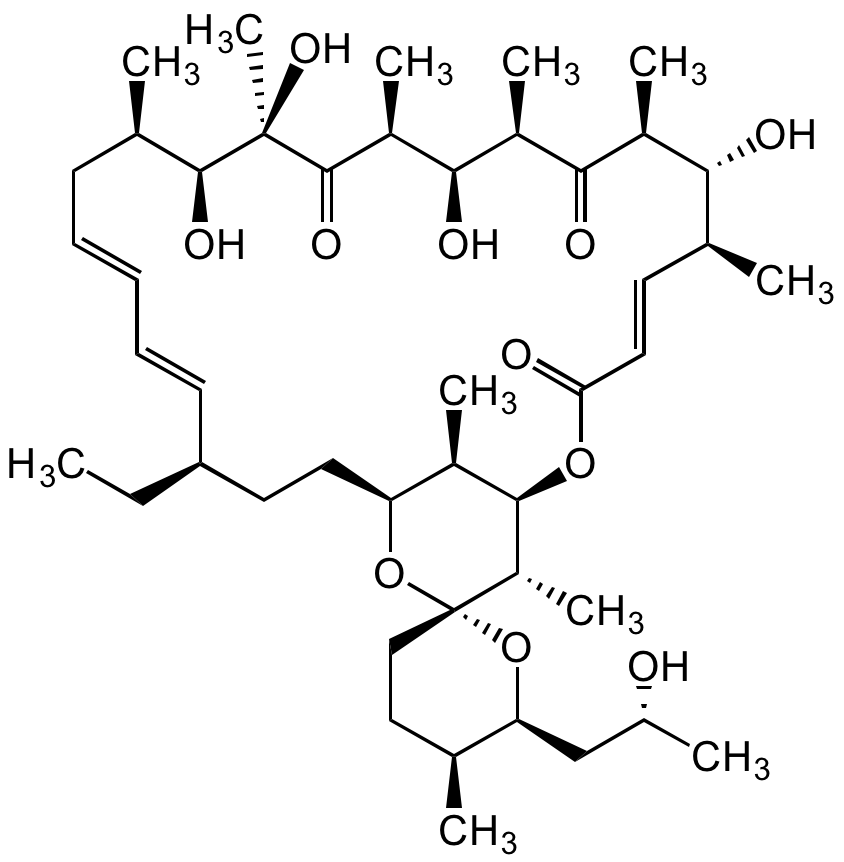
Chemical Structure
Oligomycin A [579-13-5]
AG-CN2-0517
CAS Number579-13-5
Product group Chemicals
Estimated Purity>95%
Molecular Weight791.1
Overview
- SupplierAdipoGen Life Sciences
- Product NameOligomycin A [579-13-5]
- Delivery Days Customer10
- CAS Number579-13-5
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC45H74O11
- Molecular Weight791.1
- Scientific DescriptionChemical. CAS: 579-13-5. Formula: C45H74O11. MW: 791.1. Synthetic. Originally isolated from Streptomyces diastatochromogenes. Potent mitochondrial ATP synthase (ATPases (F1F0)) inhibitor, consequently leading to inhibition of oxidative phosphorylation (OXPHOS). Useful agent for immunometabolism research. Inhibits ATP synthase by blocking its proton channel (F0 subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production), significantly reducing electron flow through the electron transport chain. Anticancer agent. Shown to induce apoptosis/necroptosis in a variety of cell types, as well as autophagy. Shown to activate AMPK. Broad antifungal, antibiotic and nematocidal agent. - Potent mitochondrial ATP synthase (ATPases (F0F1)) inhibitor, consequently leading to inhibition of oxidative phosphorylation (OXPHOS). Useful agent for immunometabolism research. Inhibits ATP synthase by blocking its proton channel (F0 subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production), significantly reducing electron flow through the electron transport chain. Anticancer agent. Shown to induce apoptosis/necroptosis in a variety of cell types, as well as autophagy. Shown to activate AMPK. Broad antifungal, antibiotic and nematocidal agent.
- SMILESC[C@H]1CC[C@@]2([C@@H](C)[C@H](OC(/C=C/[C@H](C)[C@@H](O)[C@H](C)C([C@H](C)[C@@H](O)[C@@H]3C)=O)=O)[C@H](C)[C@H](CC[C@@H](CC)/C=C/C=C/C[C@@H](C)[C@H](O)[C@@](C)(O)C3=O)O2)O[C@H]1C[C@H](O)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Oligomycin A [579-13-5]](https://www.targetmol.com/group3/M00/36/C0/CgoaEGayQySEC2aEAAAAAJyjMxs199.png)