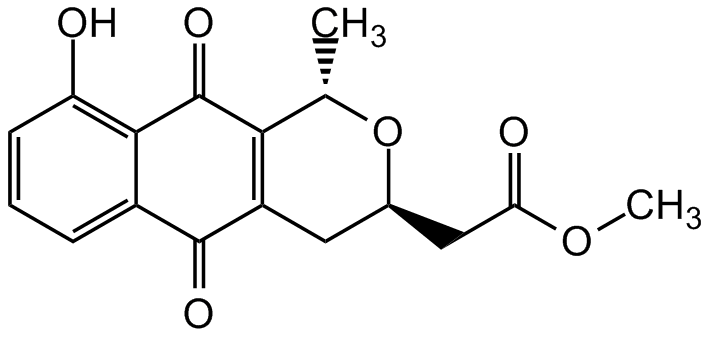
Chemical Structure
OM173-alphaA [58286-56-9]

AG-CN2-0158
Overview
- SupplierAdipoGen Life Sciences
- Product NameOM173-alphaA [58286-56-9]
- Delivery Days Customer10
- CAS Number58286-56-9
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC17H16O6
- Molecular Weight316.3
- Scientific DescriptionAntibiotic [1-3]. Antimycoplasma [1, 3, 4]. Antifungal compound [1, 3]. Anti-malarial agent [5]. Ras-competitive non-CAAX mimetic type farnesyltransferase (FTase) inhibitor [6]. Potential anticancer compound. Inducer of antiproliferative effects in tumor cell lines [6-8]. Selective DNA (cytosine-5)-methyltransferase 3B (DNMT3B) inhibitor [8]. - Chemical. CAS: 58286-56-9. Formula: C17H16O6. MW: 316.3. Isolated from Streptomyces sp. Antibiotic. Antimycoplasma. Antifungal compound. Anti-malarial agent. Ras-competitive non-CAAX mimetic type farnesyltransferase (FTase) inhibitor. Potential anticancer compound. Inducer of antiproliferative effects in tumor cell lines. Selective DNA (cytosine-5)-methyltransferase 3B (DNMT3B) inhibitor.
- SMILESCOC(=O)C[C@H]1CC2=C([C@H](C)O1)C(=O)C1=C(O)C=CC=C1C2=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Nanaomycins, new antibiotics produced by a strain of Streptomyces. I. Taxonomy, isolation, characterization and biological properties: H. Tanaka, et al.; J. Antibiot. 28, 860 (1975)
- Nanomycins, new antibiotics produced by a strain of Streptomyces. II. Structure and biosynthesis: H. Tanaka, et al.; J. Antibiot. 28, 868 (1975)
- Nanaomycins, new antibiotics produced by a strain of Streptomyces. III. A new component, nanaomycin C, and biological activities of nanaomycin derivatives: H. Tanaka, et al.; J. Antibiot. 28, 925 (1975)
- OM-173, new nanaomycin-type antibiotics produced by a strain of Streptomyces. Taxonomy, production, isolation and biological properties: Y. Iway, et al.; J. Antibiot. 36, 1268 (1983)
- Heme-dependent radical generation: possible involvement in antimalarial action of non-peroxide microbial metabolites, nanaomycin A and radicicol: Y. Tanaka, et al.; J. Antibiot. 52, 880 (1999)
- UCF76 compounds, new inhibitors of farnesyltransferase produced by Streptomyces: M. Hara, et al.; J. Antibiot. 54, 182 (2001)
- Total synthesis and development of bioactive natural products: T. Kuniaki; Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 84, 87 (2008)
- Nanaomycin A selectively inhibits DNMT3B and reactivates silenced tumor suppressor genes in human cancer cells: D. Kuck, et al.; Mol. Cancer Ther. 9, 3015 (2010)
