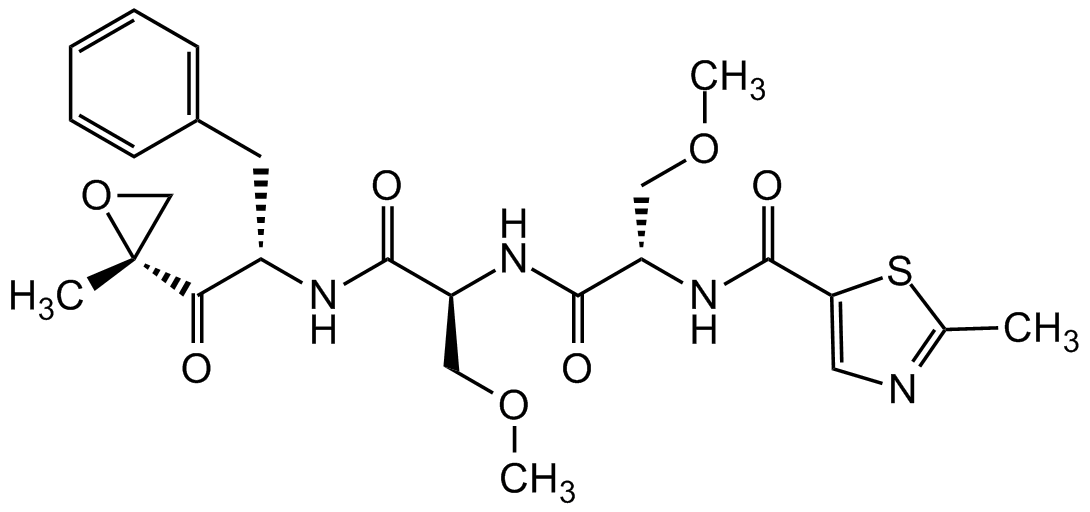
Chemical Structure
Oprozomib [ONX 0912]

AG-CR1-3672
Overview
- SupplierAdipoGen Life Sciences
- Product NameOprozomib [ONX 0912] [935888-69-0]
- Delivery Days Customer10
- CAS Number935888-69-0
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC25H32N4O7S
- Molecular Weight532.6
- Scientific DescriptionChemical. CAS: 935888-69-0. Formula: C25H32N4O7S. MW: 532.6. Synthetic. Potent orally bioavailable irreversible proteasome inhibitor. Analog of Carfilzomib (AG-CR1-3669). Targets the chymotrypsin-like beta5 subunit of the constitutive 20S proteasome (IC50=36nM) and the beta5i subunit [LMP7] of the 20S immunoproteasome (IC50=82nM). Anticancer compound effective in cell-based assays, in xenografts and against multiple myeloma in vivo. In vitro, induces cell cycle arrest and apoptosis in human cancer cell lines including multiple myeloma, as well as in bortezomib resistant multiple myeloma cells. Shown to have anti-angiogenic activity. Has potential applications in certain types of cancer as well as other diseases that require proteasome activity. - Potent orally bioavailable irreversible proteasome inhibitor. Analog of Carfilzomib (Prod. No. AG-CR1-3669). Targets the chymotrypsin-like beta5 subunit of the constitutive 20S proteasome (IC50=36nM) and the beta5i subunit [LMP7] of the 20S immunoproteasome (IC50=82nM). Anticancer compound effective in cell-based assays, in xenografts and against multiple myeloma in vivo. In vitro, induces cell cycle arrest and apoptosis in human cancer cell lines including multiple myeloma, as well as in bortezomib resistant multiple myeloma cells. Shown to have anti-angiogenic activity. Has potential applications in certain types of cancer as well as other diseases that require proteasome activity.
- SMILESO=C([C@]1(CO1)C)[C@H](CC2=CC=CC=C2)NC([C@H](COC)NC([C@H](COC)NC(C3=CN=C(C)S3)=O)=O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047): H.J. Zhou, et al.; J. Med. Chem. 52, 3028 (2009)
- A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma: D. Chauhan, et al.; Blood 116, 4906 (2010)
- The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects: M.A. Hurchla, et al.; Leukemia 27, 430 (2013)
- Carfilzomib and ONX 0912 inhibit cell survival and tumor growth of head and neck cancer and their activities are enhanced by suppression of Mcl-1 or autophagy: Y. Zang, et al.; Clin. Cancer Res. 18, 5639 (2012)
- The next generation proteasome inhibitors carfilzomib and oprozomib activate prosurvival autophagy via induction of the unfolded protein response and ATF4: Y. Zang, et al.; Autophagy 8, 1873 (2012)
- Effect of novel proteasome and immunoproteasome inhibitors on dendritic cell maturation, function, and expression of IkappaB and NFkappaB: A.S. Al-Homsi, et al.; Transpl. Immunol. 29, 1 (2013)
- Overview of proteasome inhibitor-based anti-cancer therapies: Perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system: Q.P. Dou & J.A. Zonder; Curr. Cancer Drug Targets 14, 517 (2014)
- Proteasome inhibitors - molecular basis and current perspectives in multiple myeloma: L. Kubiczkova, et al.; J. Cell. Mol. Med. 18, 947 (2014)
- Next-generation proteasome inhibitor oprozomib synergizes with modulators of the unfolded protein response to suppress hepatocellular carcinoma: Y.P. Vandewynckel, et al.; Oncotarget 7, 34988 (2016)
- Second Generation Proteasome Inhibitors in Multiple Myeloma: A. Gozzetti, et al.; Anticancer Agents Med. Chem. 17, 920 (2017)
