Chemical Structure
Penicillide [55303-92-9]

AG-CN2-0122
Overview
- SupplierAdipoGen Life Sciences
- Product NamePenicillide [55303-92-9]
- Delivery Days Customer10
- CAS Number55303-92-9
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC21H24O6
- Molecular Weight372.4
- Scientific DescriptionChemical. CAS: 55303-92-9. Formula: C21H24O6. MW: 372.4. Isolated from Penicillium sp. Plant growth inhibitor. Acyl-CoA-cholesterol acyltransferase (ACAT) inhibitor. Anticancer compound. Cytotoxic. Non-peptidic oxytocin receptor antagonist. Calpain inhibitor. - Plant growth inhibitor [1]. Mycotoxin. Acyl-CoA-cholesterol acyltransferase (ACAT) inhibitor [3, 6]. Anticancer compound. Cytotoxic [4]. Non-peptidic oxytocin receptor antagonist [5]. Calpain inhibitor [7].
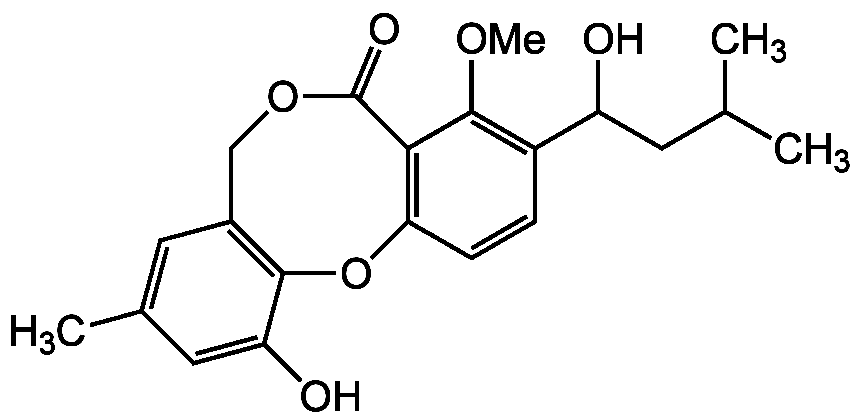
- SMILESCOC1=C(C=CC2=C1C(=O)OCC1=C(O2)C(O)=CC(C)=C1)C(O)CC(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Structure of penicillide, a new metabolite produced by a Penicillium sp.: T. Sassa, et al.; Tetrahedron Lett. 15, 3941 (1974)
- Penicillide and dehydroisopenicillide from Talaromyces derxii: K. Suzuki, et al.; Phytochemistry 30, 2096 (1991)
- Purpactins, new inhibitors of acyl-CoA:cholesterol acyltransferase produced by Penicillium purpurogenum. III. Chemical modification of purpactin A: H. Nishida, et al.; J. Antibiot. (Tokyo) 44, 152 (1991)
- Vermixocins A and B, two novel metabolites from Penicillium vermiculatum: B. Proksa, et al.; J. Antibiot. (Tokyo) 45, 1268 (1992)
- Potent, non-peptidic oxytocin receptor antagonists from a natural source: G.M. Salituro, et al.; Bioorg. Med. Chem. Lett. 3, 337 (1993)
- AS-186 compounds, new inhibitors of acyl-CoA: cholesterol acyltransferase from Penicillium asperosporum KY1635: K. Kuroda, et al.; J. Antibiot. (Tokyo) 47, 16 (1994)
- Penicillide, a Nonpeptide Calpain Inhibitor, Produced by Penicillium sp. F60760: M.C: Chung, et al.; J. Microbiol. Biotechnol. 8, 188 (1998)
