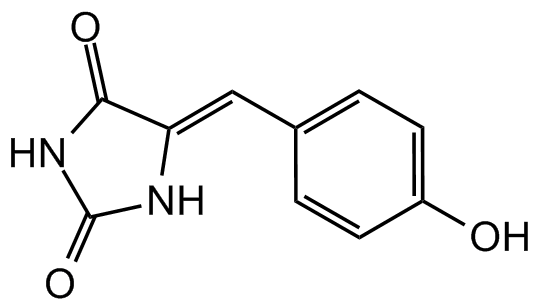
Chemical Structure
Phenylmethylene hydantoin [PMH]
AG-CN2-0041
CAS Number80171-33-1
Product group Chemicals
Estimated Purity>97%
Molecular Weight204.2
Overview
- SupplierAdipoGen Life Sciences
- Product NamePhenylmethylene hydantoin [PMH]
- Delivery Days Customer10
- CAS Number80171-33-1
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC10H8N2O3
- Molecular Weight204.2
- Scientific DescriptionAnticancer compound [2, 3, 5, 6]. Chemopreventive [2, 3, 5, 6, 7]. Shows anti-metastatic activity in prostate cancer cells through enhancement of cell-cell adhesion [2, 3]. Anti-invasive compound [5, 6, 7]. Glycogen synthase kinase-3beta (GSK-3 beta) inhibitor [4]. - Chemical. CAS: 80171-33-1. Formula: C10H8N2O3. MW: 204.2. Isolated from an unidentifed marine sponge. Anticancer compound. Chemopreventive. Shows anti-metastatic activity in prostate cancer cells through enhancement of cell-cell adhesion. Anti-invasive compound. Glycogen synthase kinase-3beta (GSK-3 beta) inhibitor.
- SMILESOC1=CC=C(\C=C2/NC(=O)NC2=O)C=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
