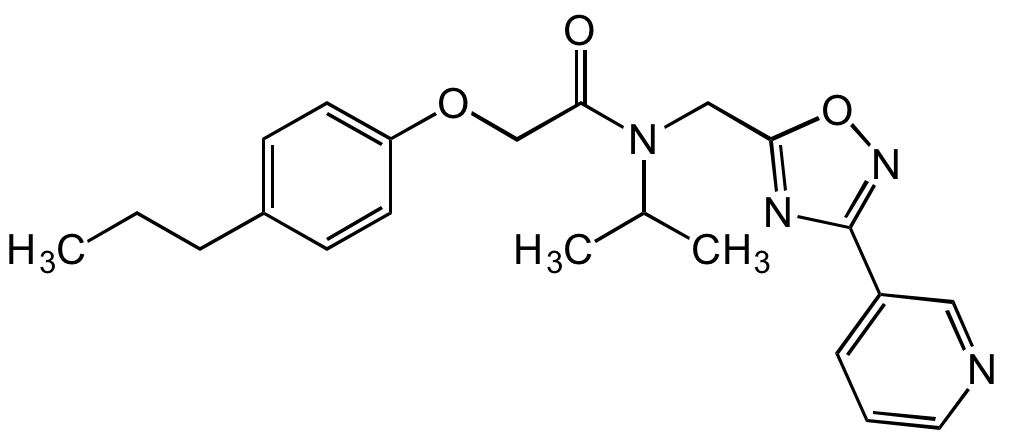
Chemical Structure
PI-1840 [Proteasome Inhibitor]

AG-CR1-3675
Overview
- SupplierAdipoGen Life Sciences
- Product NamePI-1840 [Proteasome Inhibitor] [1401223-22-0]
- Delivery Days Customer10
- CAS Number1401223-22-0
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC22H26N4O3
- Molecular Weight394.5
- Scientific DescriptionChemical. CAS: 1401223-22-0. Formula: C22H26N4O3. MW: 394.5. Synthetic. Highly potent, selective and rapidly reversible non-covalent proteasome inhibitor. Targets the chymotrypsin-like beta5-subunit of the constitutive 20S proteasome (IC50=27nM), with minimal crossreactivity on the trypsin-like (beta2) and caspase-like/postglutamyl-peptide-hydrolysis-like (beta1) proteolytic activity (IC50= >100microM, for both). Exhibited over 100-fold selectivity for the constitutive 20S proteasome over the immunoproteasome. Anticancer compound. In vitro, induces the accumulation of proteasome substrates p27, Bax, and IkappaB-alpha, inhibits survival pathways and viability and induces apoptosis in intact cancer cells. Shown to inhibit tumor growth in mice of MDA-MB-231 breast tumors. - Highly potent, selective and rapidly reversible non-covalent proteasome inhibitor. Targets the chymotrypsin-like beta5-subunit of the constitutive 20S proteasome (IC50=27nM), with minimal cross-reactivity on the trypsin-like (beta2) and caspase-like/postglutamyl-peptide-hydrolysis-like (beta1) proteolytic activity (IC50= >100microM, for both). Exhibited over 100-fold selectivity for the constitutive 20S proteasome over the immunoproteasome. Anticancer compound. In vitro, induces the accumulation of proteasome substrates p27, Bax, and IkappaB-alpha, inhibits survival pathways and viability and induces apoptosis in intact cancer cells. Shown to inhibit tumor growth in mice of MDA-MB-231 breast tumors.
- SMILESCCCC1=CC=C(OCC(N(CC2=NC(C3=CC=CN=C3)=NO2)C(C)C)=O)C=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC51202000
References
- Oxadiazole-isopropylamides as potent and noncovalent proteasome inhibitors: S. Ozcan, et al.; J. Med. Chem. 56, 3783 (2013)
- Discovery of PI-1840, a novel noncovalent and rapidly reversible proteasome inhibitor with anti-tumor activity: A. Kazi, et al.; J. Biol. Chem. 289, 11906 (2014)
