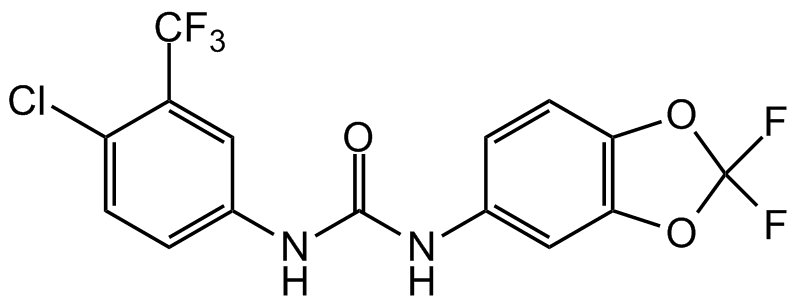
Chemical Structure
PK150 (Sorafenib Analog) [2165324-62-7]

AG-CR1-0162
CAS Number2165324-62-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight394.7
Overview
- SupplierAdipoGen Life Sciences
- Product NamePK150 (Sorafenib Analog) [2165324-62-7]
- Delivery Days Customer10
- CAS Number2165324-62-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC15H8ClF5N2O3
- Molecular Weight394.7
- Scientific DescriptionAnalog of the anticancer drug sorafenib. PK150 is a potent antibiotic. It shows oral bioavailability and antibacterial activity against several pathogenic strains at submicromolar concentrations. PK150 inhibits Gram-positive Methicillin-sensitive S. aureus (MSSA), Methicillin-resistant S. aureus (MRSA), Vancomycin intermediate S. aureus (VISA) with MICs of 0.3, 0.3-1, 0.3 microM, respectively. PK150 did not induce in vitro resistance and shows oral bioavailability and in vivo efficacy. The mode of action includes the interference with menaquinone biosynthesis by inhibiting demethylmenaquinone methyltransferase and the stimulation of protein secretion by altering the activity of signal peptidase IB. - Chemical. CAS: 2165324-62-7. Formula: C15H8ClF5N2O3. MW: 394.7. Analog of the anticancer drug sorafenib. PK150 is a potent antibiotic. It shows oral bioavailability and antibacterial activity against several pathogenic strains at submicromolar concentrations. PK150 inhibits Gram-positive Methicillin-sensitive S. aureus (MSSA), Methicillin-resistant S. aureus (MRSA), Vancomycin intermediate S. aureus (VISA) with MICs of 0.3, 0.3-1, 0.3 microM, respectively. PK150 did not induce in vitro resistance and shows oral bioavailability and in vivo efficacy. The mode of action includes the interference with menaquinone biosynthesis by inhibiting demethylmenaquinone methyltransferase and the stimulation of protein secretion by altering the activity of signal peptidase IB.
- SMILESO=C(NC1=CC(OC(F)(F)O2)=C2C=C1)NC3=CC=C(Cl)C(C(F)(F)F)=C3
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
