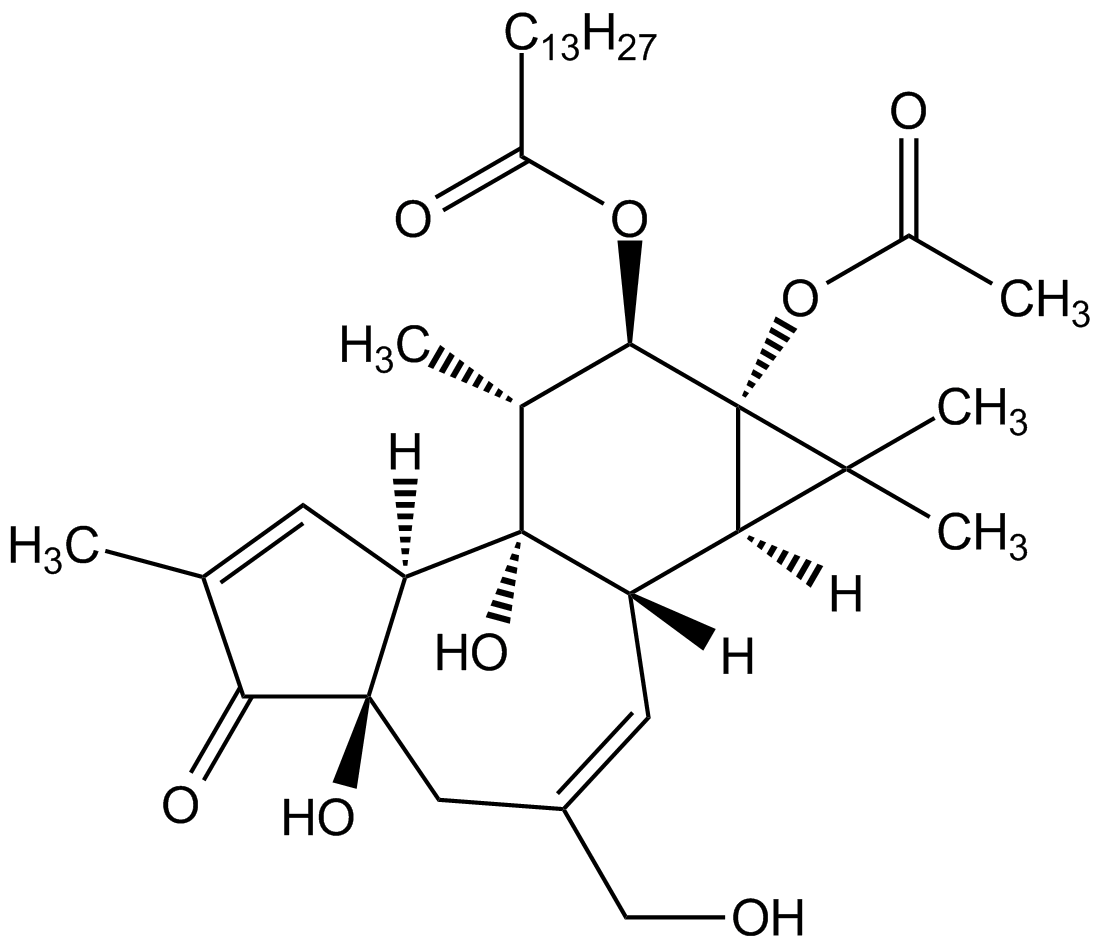
Chemical Structure
PMA [16561-29-8] [16561-29-8]
AG-CN2-0010
CAS Number16561-29-8
Product group Chemicals
Estimated Purity>98%
Molecular Weight616.8
Overview
- SupplierAdipoGen Life Sciences
- Product NamePMA [16561-29-8] [16561-29-8]
- Delivery Days Customer10
- CAS Number16561-29-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC36H56O8
- Molecular Weight616.8
- Scientific DescriptionChemical. CAS: 16561-29-8. Formula: C36H56O8. MW: 616.8. Semisynthetic. Most commonly-used phorbol ester. Binds to and activates protein kinase C (PKC) at nM concentrations. Induces cell growth arrest through a variety of pathways including the mitogen-activated protein kinases (MAPKs), p38 and c-Jun N-terminal kinase (JNK) pathways mediated by cyclin dependent kinase (CDK) inhibitors such as p21WAF1/CIP1, p27KIP1, p15 and p16. Potent mouse skin tumor promoter. Promoter of inducible NOS (iNOS; NOS II). Apoptosis inducer. Potential effective cancer therapeutic agent. Inhibitor of anti-lipolytic activity of insulin. - PMA/TPA is the most commonly-used phorbol ester [1,2]. Binds to and activates protein kinase C (PKC) at nM concentrations [1,2,9]. Induces cell growth arrest through a variety of pathways including the mitogen-activated protein kinases (MAPKs), p38 and c-Jun N-terminal kinase (JNK) pathways mediated by cyclin dependent kinase (CDK) inhibitors such as p21WAF1/CIP1, p27KIP1, p15 and p16. Potent mouse skin tumor promoter [3,4]. Promoter of inducible NOS (iNOS; NOS II) [5]. Apoptosis inducer [6]. Potential effective cancer therapeutic agent [7,8]. Inhibitor of anti-lipolytic activity of insulin [10]. PMA treatment stimulates THP-1 cells and generates M0 macrophages (M0).
- SMILES[H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)CCCCCCCCCCCCC)[C@@]1(OC(C)=O)C2(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Phorbol 12-myristate 13-acetate [16561-29-8]](https://www.targetmol.com/group3/M00/35/E0/CgoaEWayMGKEORjwAAAAACvkOcM521.png)
