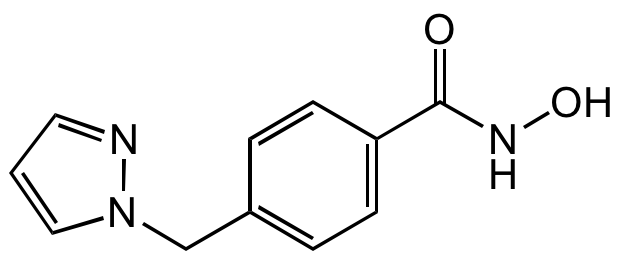
Chemical Structure
PMPH [1392835-64-1]

AG-CR1-3905
Overview
- SupplierAdipoGen Life Sciences
- Product NamePMPH [1392835-64-1]
- Delivery Days Customer10
- CAS Number1392835-64-1
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC11H11N3O2
- Molecular Weight217.2
- Scientific DescriptionCell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =11nM). Displays high selectivity over HDAC1 (IC50=1.5microM). Neuroprotective. Effectively prevents neuronal cell death upon oxidative stress induction by HCA and selectively induces cellular alpha-tubulin hyperacetylation. HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration. - Chemical. CAS: 1392835-64-1. Formula: C11H11N3O2. MW: 217.2. Cell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =11nM). Displays high selectivity over HDAC1 (IC50=1.5microM). Neuroprotective. Effectively prevents neuronal cell death upon oxidative stress induction by HCA and selectively induces cellular alpha-tubulin hyperacetylation. HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration.
- SMILESONC(=O)C1=CC=C(CN2C=CC=N2)C=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Bicyclic-capped histone deacetylase 6 inhibitors with improved activity in a model of axonal charcot-marie-tooth disease: S. Shen, et al.; ACS Chemical Neuroscience (submitted) (2015)
