Chemical Structure
Podophyllotoxin [518-28-5] [518-28-5]
AG-CN2-0049
CAS Number518-28-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight414.4
Overview
- SupplierAdipoGen Life Sciences
- Product NamePodophyllotoxin [518-28-5] [518-28-5]
- Delivery Days Customer10
- CAS Number518-28-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
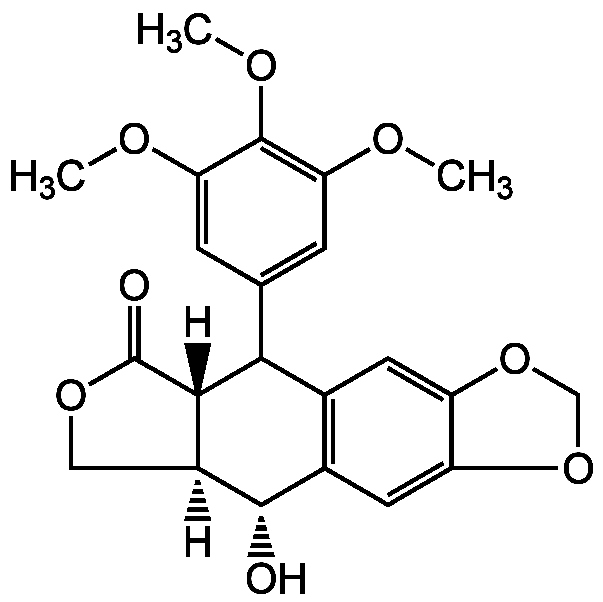
- Molecular FormulaC22H22O8
- Molecular Weight414.4
- Scientific DescriptionChemical. CAS: 518-28-5. Formula: C22H22O8. MW: 414.4. Isolated from Podophyllum emodi rhizomes. Potent microtubule assembly inhibitor. Anticancer compound. Cell death inducer. DNA topoisomerase II inhibitor. Cell cycle inhibitor at the metaphase. Antiviral and antihelminthic. - Potent microtubule assembly inhibitor [1-3]. Anticancer compound [1-5, 7]. Cell death inducer [1-5, 7]. DNA topoisomerase II inhibitor. Cell cycle inhibitor at the metaphase [2, 6, 8]. Antiviral [3, 4, 9] and antihelminthic [1].
- SMILES[H][C@]12COC(=O)[C@]1([H])C(C1=CC(OC)=C(OC)C(OC)=C1)C1=C(C=C3OCOC3=C1)[C@@H]2O
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200

![Podofilox [518-28-5] [518-28-5]](https://www.targetmol.com/group3/M00/03/6A/CgoaEWY7SvuEIN-aAAAAAAW5qOA528.png)