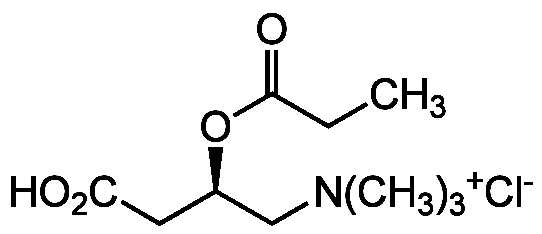
Chemical Structure
Propionyl-L-carnitine . hydrochloride
AG-CR1-3595
CAS Number119793-66-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight217.3 . 36.5
Overview
- SupplierAdipoGen Life Sciences
- Product NamePropionyl-L-carnitine . hydrochloride
- Delivery Days Customer10
- CAS Number119793-66-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC10H19NO4 . HCl
- Molecular Weight217.3 . 36.5
- Scientific DescriptionChemical. CAS: 119793-66-7. Formula: C10H19NO4 . HCl. MW: 217.3 . 36.5. Synthetic. Naturally occurring carnitine derivative formed by carnitine acetyltransferase during beta-oxidation of uneven chain fatty acids, with high affinity for muscular carnitine transferase. Increases cellular carnitine content, allowing free fatty acid transport into the mitochondria. Stimulates energy production in ischaemic muscles by increasing citric acid cycle flux and stimulating pyruvate dehydrogenase activity. Important for mitochondrial metabolism and energy regulation. Regulates the metabolism of both carbohydrates and lipids, leading to an increase of ATP generation. Selectively inhibits in vitro and ex vivo platelet-activating factor (PAF) synthesis from human neutrophils. Antioxidant. Shows free radical scavenging activity. Decreases the expression of inducible nitric oxide synthase (iNOS/NOS II) and NADPH-oxidase 4-mediated reactive oxygen species production in human umbilical vascular endothelial cells. Shows beneficial cardiovascular effects. Improves body weight, food intake, adiposity and insulin resistance in Type 2 diabetes. Stimulates endothelial nitric oxide (eNOS/NOS III) and increased NO production, via AMPK/Src-mediated signaling that leads to activation of PI3 kinase and Akt. - Naturally occurring carnitine derivative formed by carnitine acetyltransferase during beta-oxidation of uneven chain fatty acids, with high affinity for muscular carnitine transferase. Increases cellular carnitine content, allowing free fatty acid transport into the mitochondria [1, 5]. Stimulates energy production in ischaemic muscles by increasing citric acid cycle flux and stimulating pyruvate dehydrogenase activity [1, 2]. Important for mitochondrial metabolism and energy regulation. Regulates the metabolism of both carbohydrates and lipids, leading to an increase of ATP generation [9, 11]. Selectively inhibits in vitro and ex vivo platelet-activating factor (PAF) synthesis from human neutrophils [3]. Antioxidant. Shows free radical scavenging activity [4]. Decreases the expression of inducible nitric oxide synthase (iNOS/NOS II) and NADPH-oxidase 4-mediated reactive oxygen species production in human umbilical vascular endothelial cells [7]. Shows beneficial cardiovascular effects. Improves body weight, food intake, adiposity and insulin resistance in Type 2 diabetes [5, 6, 8-10]. Stimulates endothelial nitric oxide (eNOS/NOS III) and increased NO production, via AMPK/Src-mediated signaling that leads to activation of PI3 kinase and Akt [6, 12].
- SMILESCC[C@@H](CC(O)=O)OC(=O)CC
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Levocarnitine propionate hydrochloride [119793-66-7]](https://www.targetmol.com/group3/M00/02/49/CgoaEGY7LsiEbnpyAAAAAJUPy2E141.png)
