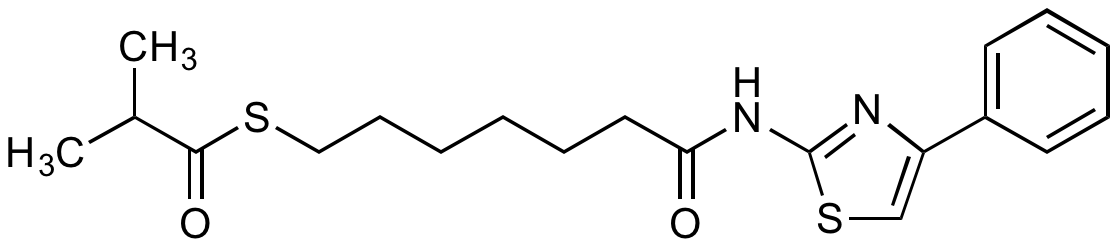
Chemical Structure
PTACH [NCH 51]

AG-CR1-3667
Overview
- SupplierAdipoGen Life Sciences
- Product NamePTACH [NCH 51] [848354-66-5]
- Delivery Days Customer10
- CAS Number848354-66-5
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationDanger
- Molecular FormulaC20H26N2O2S2
- Molecular Weight390.56
- Scientific DescriptionChemical. CAS: 848354-66-5. Formula: C20H26N2O2S2. MW: 390.56. Synthetic. Potent non-hydroxamate HDAC inhibitor (HDACi) (IC50: 32, 48 and 41nM for HDAC4, HDAC1 and HDAC6, respectively). Cell-permeable prodrug that is intracellularly converted to the potent HDAC inhibitor NCH 31. Predicted to exhibit a similar HDAC binding mode as that of SAHA, interacting with the active-site zinc targeting group. Shown to exhibit comparable antiproliferative and apoptotic activity as SAHA against various cancer cell lines. Inhibits growth of various cancer cells in vitro (EC50 = 1.1 - 9.1mM). Reactivates latent HIV-1 gene expression. Active against selected neurodevelopmental disorders. - Potent non-hydroxamate HDAC inhibitor (HDACi) (IC50: 32, 48 and 41nM for HDAC4, HDAC1 and HDAC6, respectively). Cell permeable prodrug that is intracellularly converted to the potent HDAC inhibitor NCH 31. Predicted to exhibit a similar HDAC binding mode as that of SAHA, interacting with the active-site zinc targeting group. Shown to exhibit comparable antiproliferative and apoptotic activity as SAHA against various cancer cell lines. Inhibits growth of various cancer cells in vitro (EC50=1.1 - 9.1microM). Reactivates latent HIV-1 gene expression. Active against selected neurodevelopmental disorders.
- SMILESCC(C)C(=O)SCCCCCCC(=O)NC1=NC(=CS1)C1=CC=CC=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Novel inhibitors of human histone deacetylases: design, synthesis, enzyme inhibition, and cancer cell growth inhibition of SAHA-based non-hydroxamates: T. Suzuki, et al.; J. Med. Chem. 48, 1019 (2005)
- Proteome analyses of the growth inhibitory effects of NCH-51, a novel histone deacetylase inhibitor, on lymphoid malignant cells: T. Sanda, et al.; Leukemia 21, 2344 (2007)
- Identification of a potent and stable antiproliferative agent by the prodrug formation of a thiolate histone deacetylase inhibitor: T. Suzuki, et al.; Bioorg. Med. Chem. Lett. 17, 1558 (2007)
- Novel histone deacetylase inhibitor NCH-51 activates latent HIV-1 gene expression: A.F. Victoriano, et al.; FEBS Lett. 585, 1103 (2011)
- Immunological and pharmacological strategies to reactivate HIV-1 from latently infected cells: a possibility for HIV-1 paediatric patients? M. Martinez-Bonet, et al.; J. Virus Erad. 1, 148 (2015)
- Altered neuronal network and rescue in a human MECP2 duplication model: S. Nageshappa, et al.; Mol. Psychiatry 21, 178 (2016)
