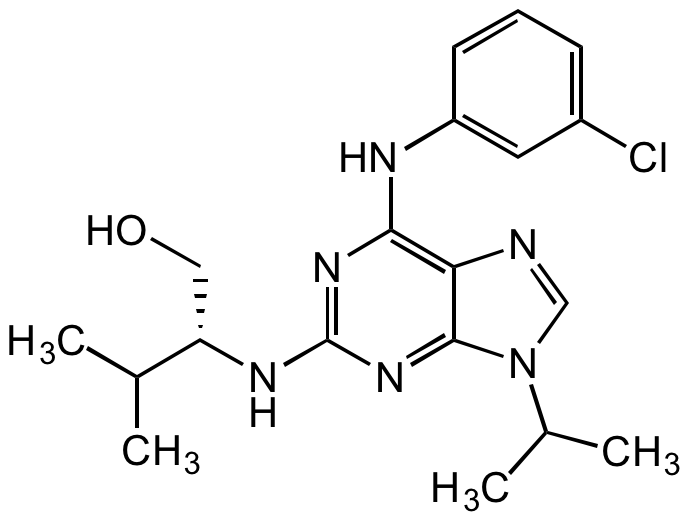
Chemical Structure
Purvalanol A [212844-53-6]

AG-CR1-2903
Overview
- SupplierAdipoGen Life Sciences
- Product NamePurvalanol A [212844-53-6]
- Delivery Days Customer10
- CAS Number212844-53-6
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC19H25ClN6O
- Molecular Weight388.9
- Scientific DescriptionChemical. CAS: 212844-53-6. Formula: C19H25ClN6O. MW: 388.9. Synthetic. Potent, cell permeable cyclin-dependent protein kinase (cdk) inhibitor. Inhibits human CDK1 (IC50=4nM), CDK2/cyclin A (IC50=70nM), Cdc2/cyclin B, CDK2/cyclin E (IC50=35nM), CDK4/cyclin D1 (IC50=850nM) as well as CDK5/p35 (IC50=75nM). DYRK1A inhibitor (IC50=300nM). Anticancer agent. Strong apoptotic and autophagy inducer which causes cell cycle arrest in the G1 and G2 phase in various cancer cell lines. More membrane permeable than purvalanol B. Shown to inhibit ABCB1 and ABCB2 transporters and useful in co-treatment with selected anticancer drugs. - Potent, cell permeable cyclin-dependent protein kinase (cdk) inhibitor. Inhibits human CDK1 (IC50=4nM), CDK2/cyclin A (IC50=70nM), Cdc2/cyclin B, CDK2/cyclin E (IC50=35nM), CDK4/cyclin D1 (IC50=850nM) as well as CDK5/p35 (IC50=75nM). DYRK1A inhibitor (IC50=300nM). Anticancer agent. Strong apoptotic and autophagy inducer which causes cell cycle arrest in the G1 and G2 phase in various cancer cell lines. More membrane permeable than purvalanol B. Shown to inhibit ABCB1 and ABCB2 transporters and useful in co-treatment with selected anticancer drugs.
- SMILESClC1=CC=CC(NC2=C3C(N(C(C)C)C=N3)=NC(N[C@@H](CO)C(C)C)=N2)=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC51202000
References
- Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors: N.S. Gray, et al.; Science 281, 533 (1998)
- The specificities of protein kinase inhibitors: An update: J. Bain, et al.; Biochem. J. 371, 199 (2003)
- Cellular effects of purvalanol A: A specific inhibitor of cyclin-dependent kinase activities: N. Villerbu, et al.; Int. J. Cancer 97, 761 (2002)
- Purvalanol A induces apoptosis and downregulation of antiapoptotic proteins through abrogation of phosphorylation of JAK2/STAT3 and RNA polymerase II: D. Iizuka, et al.; Anticancer Drugs 19, 565 (2008)
- Purvalanol A, a CDK inhibitor, effectively suppresses Src-mediated transformation by inhibiting both CDKs and c-Src: T. Hikita, et al.; Genes to Cells 15, 1051 (2010)
- Olomoucine II and purvalanol A inhibit ABCG2 transporter in vitro and in situ and synergistically potentiate cytostatic effect of mitoxantrone: J. Hofman, et al.; Pharmacol. Res. 65, 312 (2012)
- Purvalanol A, olomoucine II and roscovitine inhibit ABCB1 transporter and synergistically potentiate cytotoxic effects of daunorubicin in vitro: D. Cihalova, et al.; PLoS One 8, e83467 (2013)
- Purvalanol induces endoplasmic reticulum stress-mediated apoptosis and autophagy in a time-dependent manner in HCT116 colon cancer cells: A. Coker-Gurkan, et al.; Oncol. Rep. 33, 2761 (2015)
