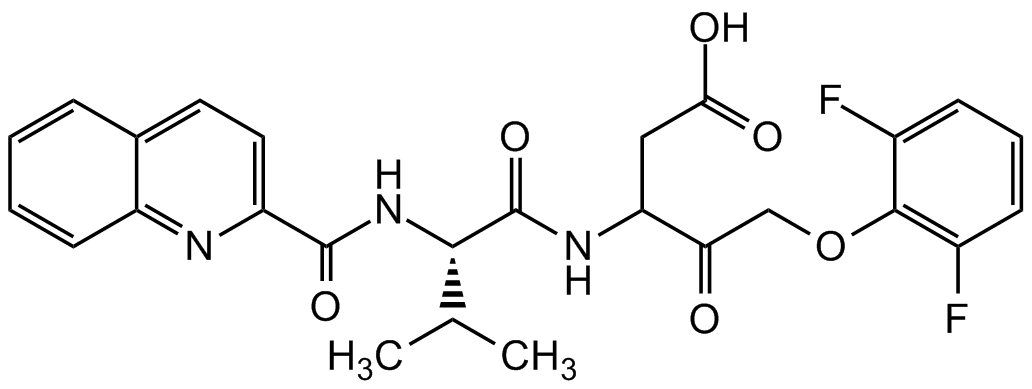
Chemical Structure
Q-VD-OPh [1135695-98-5]

AG-CP3-0006
Overview
- SupplierAdipoGen Life Sciences
- Product NameQ-VD-OPh [1135695-98-5]
- Delivery Days Customer10
- CAS Number1135695-98-5
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC26H25F2N3O6
- Molecular Weight513.5
- Scientific DescriptionCell permeable, irreversible and non-toxic non-FMK pan-caspase inhibitor with improved potency, stability and toxicity over Z-VAD-FMK. Does not cross-react with cathepsins nor calpains. Non-toxic due to the 2,6-difluorophenoxy methyl (OPh) group. The peptide is not O-methylated to reduce hydrophobicity and to facilitate use in aqueous media. Inhibits ICE-family protease/caspase processing, leading to apoptosis and autophagy induction. Decreases proteasome activity. Used in apoptosis and inflammasome studies. - Chemical. CAS: 1135695-98-5 (anhydrous). Formula: C26H25F2N3O6. MW: 513.5. Synthetic. Cell permeable, irreversible and non-toxic non-FMK pan-caspase inhibitor with improved potency, stability and toxicity over Z-VAD-FMK. Does not cross-react with cathepsins nor calpains. Non-toxic due to the 2,6-difluorophenoxy methyl (OPh) group. The peptide is not O-methylated to reduce hydrophobicity and to facilitate use in aqueous media. Inhibits ICE-family protease/caspase processing, leading to apoptosis and autophagy induction. Decreases proteasome activity. Used in apoptosis and inflammasome studies.
- SMILESCC(C)[C@H](NC(=O)C1=CC=C2C=CC=CC2=N1)C(=O)NC(CC(O)=O)C(=O)COC1=C(F)C=CC=C1F
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties: T.M. Caserta, et al.; Apoptosis 8, 345 (2003)
- Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender: S. Renolleau, et al.; J. Neurochem. 100, 1062 (2007)
- The role of caspases in Alzheimer's disease; potential novel therapeutic opportunities: T.T. Rohn; Apoptosis 15, 1403 (2010)
- The pan-caspase inhibitor Q-VD-OPh has anti-leukemia effects and can interact with vitamin D analogs to increase HPK1 signaling in AML cells: X. Chen-Deutsch, et al.; Leuk. Res. 36, 884 (2012)
