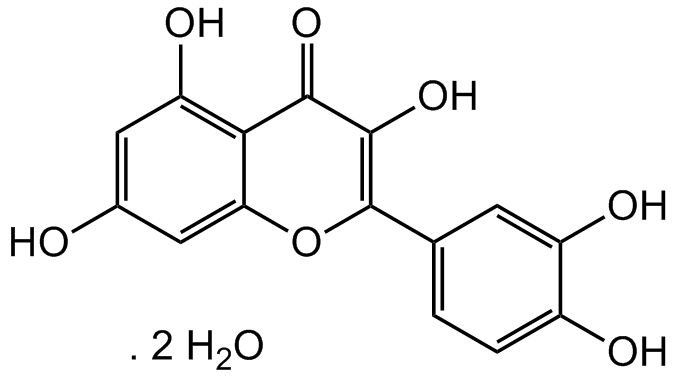
Chemical Structure
Quercetin . dihydrate [6151-25-3]

AG-CN2-0409
CAS Number6151-25-3
Product group Chemicals
Estimated Purity>95%
Molecular Weight302.2 . 36.0
Overview
- SupplierAdipoGen Life Sciences
- Product NameQuercetin . dihydrate [6151-25-3]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number6151-25-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC15H10O7 . 2H20
- Molecular Weight302.2 . 36.0
- Scientific DescriptionChemical. CAS: 6151-25-3. Formula: C15H10O7 . 2H20. MW: 302.2 . 36.0. Isolated from Umbelliferae sp. Multipotent flavonoid antioxidant. Potent free radical scavenger. Neuroprotective. Anticancer compound with chemosensitizing activity. Cell cycle arrest, apoptosis and autophagy inducer. Proteasome inhibitor. Pleiotropic kinase inhibitor, including tyrosine protein kinase (Trk), mitochondrial ATPase, cAMP- and cGMP-phosphodiesterases, PI3-kinase activity, phospholipase A2 and protein kinase C (PKC). DNA topoisomerases inhibitor. SIRT1 activator. Heat shock proteins inhibitor. Reversible fatty acid synthase inhibitor. Antithrombotic, antihistaminic and anti-inflammatory agent. Monoamine oxidase inhibitor. Vasodilatory compound. anti-diabetic compound. - Multipotent flavonoid antioxidant. Potent free radical scavenger. Neuroprotective. Anticancer compound with chemosensitizing activity. Cell cycle arrest, apoptosis and autophagy inducer. Proteasome inhibitor. Pleiotropic kinase inhibitor, including tyrosine protein kinase (Trk), mitochondrial ATPase, cAMP- and cGMP-phosphodiesterases, PI3-kinase activity, phospholipase A2 and protein kinase C (PKC). DNA topoisomerases inhibitor. SIRT1 activator. Heat shock proteins inhibitor. Reversible fatty acid synthase inhibitor. Antithrombotic, antihistaminic and anti-inflammatory agent. Monoamine oxidase inhibitor. Vasodilatory compound. Anti-diabetic compound. Antiviral for the prevention and treatment of SARS-CoV-2 related disease (COVID-19)
- SMILESOC1=CC(O)=C2C(OC(=C(O)C2=O)C2=CC=C(O)C(O)=C2)=C1
- Storage Instruction2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200
References
- Pharmacological inhibitors of Fatty Acid Synthase (FASN) - catalyzed endogenous fatty acid biogenesis: a new family of anti-cancer agents? R. Lupu & J.A. Menendez; Curr. Pharm. Biotechnol. 7, 483 (2006) (Review)
- Health effects of quercetin: from antioxidant to nutraceutical: A.W. Boots, et al.; Eur. J. Pharmacol. 585, 325 (2008) (Review)
- Multitargeted cancer prevention by quercetin: A. Murakami, et al.; Cancer Lett. 269, 315 (2008) (Review)
- Inhibiting the transcription factor HSF1 as an anticancer strategy: L. Whitesell & S. Lindquist; Expert Opin. Ther. Targets 13, 469 (2009) (Review)
- In vitro modulation of naturally occurring flavonoids on cytochrome P450 2A6 (CYP2A6) activity: K.H. Tiong, et al.; Xenobiotica 40, 458 (2010) (Review)
- Regulation of SIRT1 in cellular functions: role of polyphenols: S. Chung, et al.; Arch. Biochem. Biophys. 501, 79 (2010) (Review)
- Quercetin as a systemic chemopreventative agent: structural and functional mechanisms: E.E. Mendoza & R. Burd; Mini Rev. Med. Chem. 11, 1216 (2011) (Review)
- Exploiting tyrosinase expression and activity in melanocytic tumors: quercetin and the central role of p53: A.J. Vargas, et al.; Integr. Cancer Ther. 10, 328 (2011) (Review)
- Targeting tumor ubiquitin-proteasome pathway with polyphenols for chemosensitization: M. Shen, et al.; Anticancer Agents Med. Chem. 12, 891 (2012) (Review)
- Flavonoids acting on DNA topoisomerases: recent advances and future perspectives in cancer therapy: P. Russo, et al.; Curr. Med. Chem. 19, 5287 (2012) (Review)
