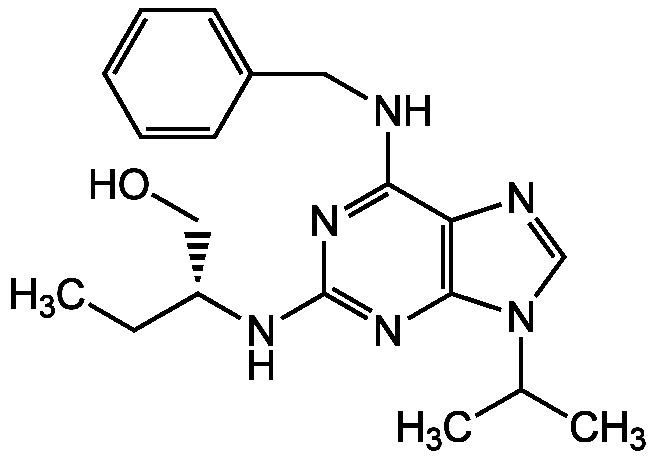
Chemical Structure
(R)-Roscovitine [186692-46-6]

AG-MR-C0001
Overview
- SupplierAdipoGen Life Sciences
- Product Name(R)-Roscovitine [186692-46-6]
- Delivery Days Customer10
- CAS Number186692-46-6
- CertificationResearch Use Only
- Estimated Purity>99%
- Molecular FormulaC19H26N6O
- Molecular Weight354.5
- Scientific DescriptionChemical. CAS: 186692-46-6. Formula: C19H26N6O. MW: 354.5. Potent and selective inhibitor of cyclin dependent kinases CDK1, CDK2, CDK5, CDK7 and CDK9. Pyridoxal kinase (PDXK) inhibitor. Triggers cell apoptotic cell death. Down-regulates Mcl-1 and MYCN. Anticancer compound. Kills chronic lymphocytic leukemia (LLC) cells and slows tumor growth in mouse xenografts. Inhibits cysts formation in culture and in polycystic kidney disease (PKD) mouse models. Potential anti-inflammatory compound that can influence the resolution of inflammation. Potential antidiabetic compound. Shown to protect pancreatic beta-cells from glucotoxicity and increase insulin secretion. Shows antiviral properties. Neuroprotective in brain trauma. Has positive effects on Timothy syndrome cells. Used for cloning of mammals by synchronization of nucleus donor cells. Provides neuroprotection in experimental traumatic brain injury. - Potent and selective inhibitor of cyclin dependent kinases CDK1, CDK2, CDK5, CDK7 and CDK9. Pyridoxal kinase (PDXK) inhibitor. Triggers cell apoptotic cell death. Down-regulates Mcl-1 and MYCN. Anticancer compound. Kills chronic lymphocytic leukemia (LLC) cells and slows tumor growth in mouse xenografts. Inhibits cysts formation in culture and in polycystic kidney disease (PKD) mouse models. Potential anti-inflammatory compound that can influence the resolution of inflammation. Potential antidiabetic compound. Shown to protect pancreatic beta-cells from glucotoxicity and increase insulin secretion. Shows antiviral properties. Neuroprotective in brain trauma. Has positive effects on Timothy syndrome cells. Used for cloning of mammals by synchronization of nucleus donor cells. Provides neuroprotection in experimental traumatic brain injury.
- SMILESCC[C@H](CO)NC1=NC2=C(N=CN2C(C)C)C(NCC2=CC=CC=C2)=N1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC51202000
References
- Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5: L. Meijer, et al.; Eur. J. Biochem. 243, 527 (1997)
- Chemical inhibitors of cyclin-dependent kinases: L. Meijer and S.H. Kim; Meth. Enzymol. 283, 113 (1997)
- Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine: WF. De Azevedo, et al.; Eur. J. Biochem. 243, 518 (1997)
- Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of Herpes simplex virus and Human immunodeficiency virus type 1 by targeting cellular, not viral, proteins: L.M. Schang, et al.; J. Virol. 76, 7874 (2002)
- Enhanced survivability of cloned calves derived from roscovitine-treated adult somatic cells: J. Gibbons, et al.; Biol. Reprod. 66, 895 (2002)
- Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials: L. Meijer & E. Raimond; Acc. Chem. Res. 36, 417 (2003)
- Crystal structure of pyridoxal kinase in complex with roscovitine and derivatives: L. Tang, et al.; J. Biol. Chem. 280, 31220 (2005)
- Roscovitine targets, protein kinases and pyridoxal kinase: S. Bach, et al.; J. Biol. Chem. 280, 31208 (2005)
- Inhibition of cyclin-dependent kinase 5 activity protects pancreatic beta cells from glucotoxicity: M. Ubeda, et al.; J. Biol. Chem. 281, 28858 (2006)
- Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis: A.G. Rossi, et al.; Nature Med. 12, 1056 (2006)
