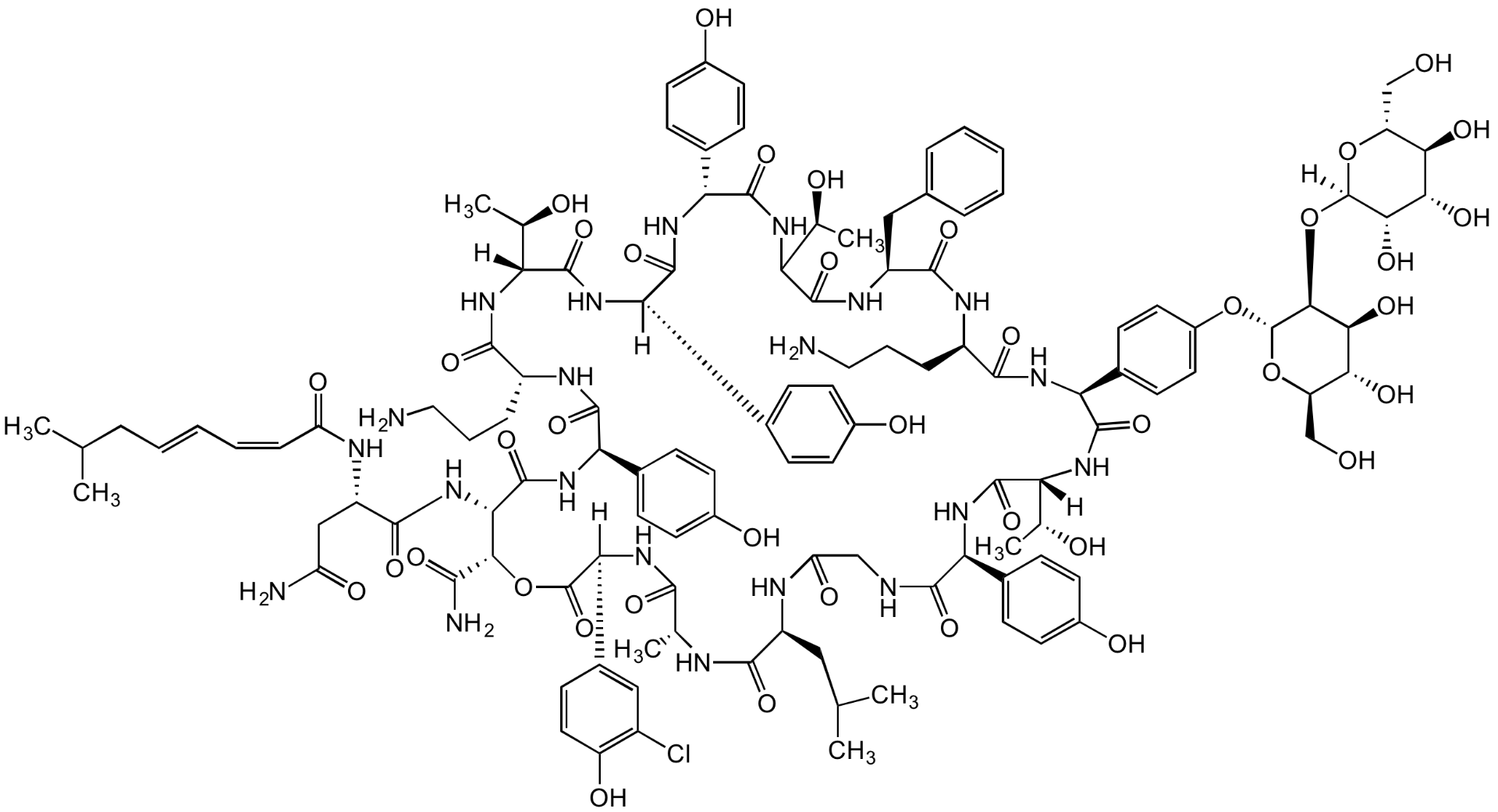
Chemical Structure
Ramoplanin A2 [81988-88-7] [81988-88-7]
AG-CN2-0318
CAS Number81988-88-7
Product group Chemicals
Estimated Purity>90%
Molecular Weight2554.1
Overview
- SupplierAdipoGen Life Sciences
- Product NameRamoplanin A2 [81988-88-7] [81988-88-7]
- Delivery Days Customer10
- CAS Number81988-88-7
- CertificationResearch Use Only
- Estimated Purity>90%
- Molecular FormulaC119H154ClN21O40
- Molecular Weight2554.1
- Scientific DescriptionChemical. CAS: 81988-88-7 [A2]. Formula: C119H154ClN21O40. MW: 2554.1. Isolated from Actinoplanes sp. Glycolipodepsipeptide antibiotic. Complex of structurally related molecules A1, A2 and A3, with ramoplanin A2 as the primary component. Antibacterial and antiviral agent with activity against aerobic and anaerobic Gram-positive bacteria such as Clostridium difficile and antibiotic-resistant enterococci. Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis. - Glycolipodepsipeptide antibiotic. Complex of structurally related molecules A1, A2 and A3, with ramoplanin A2 as the primary component. Antibacterial and antiviral agent with activity against aerobic and anaerobic Gram-positive bacteria such as Clostridium difficile and antibiotic-resistant enterococci. Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis.
- SMILESOC[C@H]([C@@H](O)[C@H](O)[C@@H]1O)O[C@]1([H])O[C@@H]2[C@H](O[C@H](CO)[C@@H](O)[C@@H]2O)OC3=CC=C([C@@H]4NC([C@H](NC([C@@H](NC(C(NC([C@@H](C5=CC=C(O)C=C5)NC([C@@](C6=CC=C(O)C=C6)([H])NC([C@@](NC([C@H](NC([C@@H](C7=CC=C(O)C=C7)NC([C@@H](NC([C@H](CC(N)=O)NC(/C=C\C=C\CC(C)C)=O)=O)[C@@H](C(N)=O)OC([C@@](C8=CC(Cl)=C(O)C=C8)([H])NC([C@@H](C)NC([C@H](CC(C)C)NC(CNC([C@H](C9=CC=C(O)C=C9)NC([C@]([C@H](O)C)([H])NC4=O)=O)=O)=O)=O)=O)=O)=O)=O)CCCN)=O)([H])[C@H](O)C)=O)=O)=O)[C@@H](O)C)=O)CC%10=CC=CC=C%10)=O)CCCN)=O)C=C3
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
