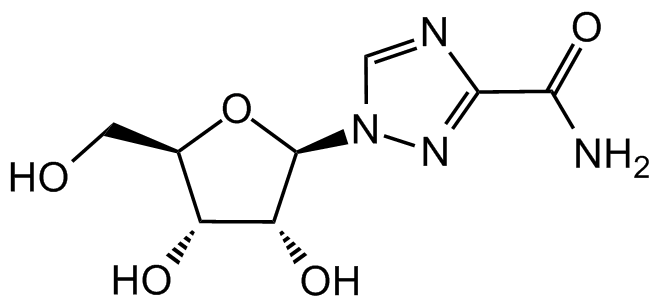
Chemical Structure
Ribavirin [36791-04-5]

AG-CR1-3719
CAS Number36791-04-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight244.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameRibavirin [36791-04-5]
- Delivery Days Customer10
- CAS Number36791-04-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC8H12N4O5
- Molecular Weight244.2
- Scientific DescriptionChemical. CAS: 36791-04-5. Formula: C8H12N4O5. MW: 244.2. Ribavirin is a guanosine analog with broad-spectrum antiviral properties against DNA and RNA viruses, including coronavirus SARS, respiratory syncytial virus, several hemorrhagic fever viruses, hepatitis C (HCV) and influenza. It is also tested for SARS-CoV-2. It acts as a prodrug that can be activated by either mono- or tri-phosphorylation by cellular kinases. These phosphorylated derivatives of ribavirin have diverse effects on both cellular and viral enzymes, resulting in suppression of viral replication and propagation. There are 3 direct mechanisms of action, i) by inhibition of the viral RNA dependent RNA polymerase (RdRp) and RNA synthesis through direct interaction with ribavirin-5-triphosphate, ii) by interfering with RNA capping activity, and iii) by increasing viral mutation rates through the misincorporation of RBV into the genome. In addition there are also 2 proposed indirect mechanisms, iv) inhibition of inosine monophosphate dehydrogenase (IMPDH) by ribavirin-5-monophosphate, which leads to the depletion of intracellular GTP pools, v) unphosphorylated Ribavirin has immunomodulatory effects on antiviral cellular responses, enhancing the T-helper type 1 over type 2 responses or upregulating the interferon-stimulated response element. Ribavirin shows anticancer properties. The principal target in several cancers is the oncogenic eukaryotic translation initiation factor 4E (eIF4E), modulated via direct binding and competitive inhibition. Ribavirin has been shown to bind eIF4E at the functional site used to bind the m7G cap of mRNA transcripts in vitro. It competes with target mRNAs for binding, subsequent translocation to the cytoplasm and ultimately translation. Other studies also demonstrate modulation of several other key pathways including ERK, EZH2 and IMPDH. - Ribavirin is a guanosine analog with broad-spectrum antiviral properties against DNA and RNA viruses, including coronavirus SARS, respiratory syncytial virus, several hemorrhagic fever viruses, hepatitis C (HCV) and influenza. It is also tested for SARS-CoV-2. It acts as a prodrug that can be activated by either mono- or tri-phosphorylation by cellular kinases. These phosphorylated derivatives of ribavirin have diverse effects on both cellular and viral enzymes, resulting in suppression of viral replication and propagation. There are 3 direct mechanisms of action, i) by inhibition of the viral RNA dependent RNA polymerase (RdRp) and RNA synthesis through direct interaction with ribavirin-5-triphosphate, ii) by interfering with RNA capping activity, and iii) by increasing viral mutation rates through the misincorporation of RBV into the genome. In addition there are also 2 proposed indirect mechanisms, iv) inhibition of inosine monophosphate dehydrogenase (IMPDH) by ribavirin-5-monophosphate, which leads to the depletion of intracellular GTP pools, v) unphosphorylated Ribavirin has immunomodulatory effects on antiviral cellular responses, enhancing the T-helper type 1 over type 2 responses or upregulating the interferon-stimulated response element. Ribavirin shows anticancer properties. The principal target in several cancers is the oncogenic eukaryotic translation initiation factor 4E (eIF4E), modulated via direct binding and competitive inhibition. Ribavirin has been shown to bind eIF4E at the functional site used to bind the m7G cap of mRNA transcripts in vitro. It competes with target mRNAs for binding, subsequent translocation to the cytoplasm and ultimately translation. Other studies also demonstrate modulation of several other key pathways including ERK, EZH2 and IMPDH.
- SMILESO[C@@H]1[C@@H](CO)O[C@@H](N2N=C(C(N)=O)N=C2)[C@@H]1O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
