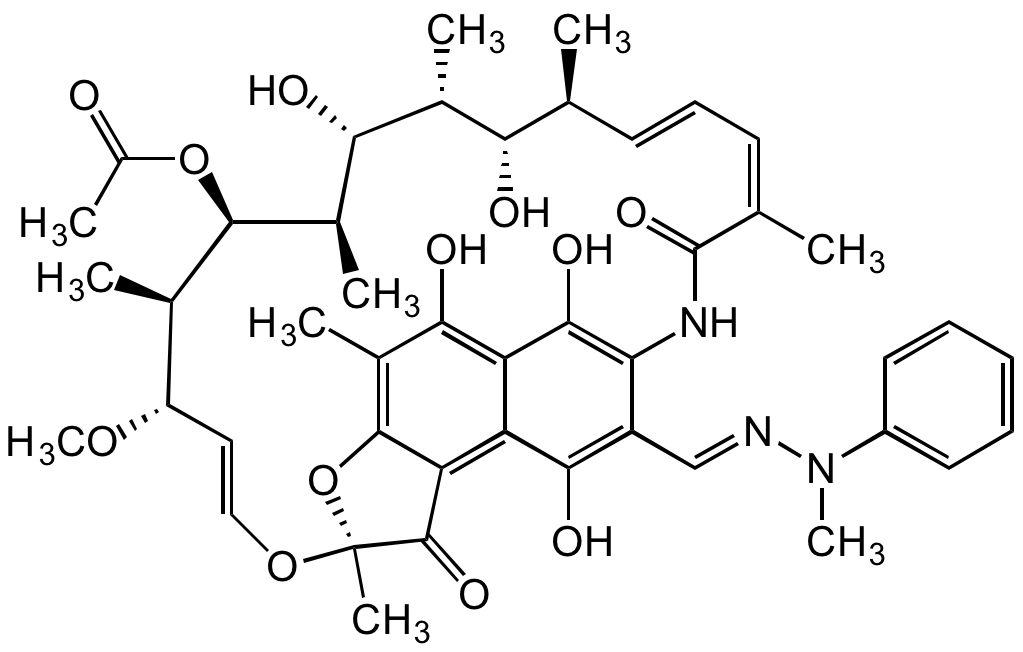
Chemical Structure
Rifamycin AF-K91725 [13292-38-1]

AG-CN2-0327
Overview
- SupplierAdipoGen Life Sciences
- Product NameRifamycin AF-K91725 [13292-38-1]
- Delivery Days Customer10
- CAS Number13292-38-1
- CertificationResearch Use Only
- Estimated Purity>90%
- Molecular FormulaC45H55N3O12
- Molecular Weight829.9
- Scientific DescriptionAnsamycin antibiotic. Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP). Effective against mycobacteria and therefore used in research of tuberculosis, leprosy and Mycobacterium avium complex (MAC) infections. - Chemical. CAS: 13292-38-1. Formula: C45H55N3O12. MW: 829.9. Semisynthetic. Ansamycin antibiotic. Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP). Effective against mycobacteria, and are therefore used in research of tuberculosis, leprosy and mycobacterium avium complex (MAC) infections.
- SMILESOC1=C(NC(/C(C)=C\C=C\[C@H](C)[C@H](O)[C@@H](C)[C@H]([C@H]2C)O)=O)C(/C=N/N(C)C3=CC=CC=C3)=C(O)C4=C5C(O[C@@](O/C=C/[C@H](OC)[C@H]([C@H]2OC(C)=O)C)(C)C5=O)=C(C)C(O)=C41
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC51280000
References
- Rifamycin antibiotics: inhibitors of Rauscher murine leukemia virus reverse transcriptase and of purified DNA polymerases from human normal and leukemic lymphoblasts: S.S. Yang, et al.; J. Natl. Cancer Inst. 49, 7 (1972)
- Rifamycin Derivatives Strongly Inhibiting RNA>DNA Polymerase (Reverse Transcriptase) of Murine Sarcoma Viruses: C. Gurgo, et al.; J. Natl. Cancer Inst. 49, 61 (1972)
- Hydrazones of 3-formylrifamycin SV. III. N-(Mono and di)substituted hydrazone derivatives. Synthesis, antibacterial activity, and other biological properties: R. Cricchio, et al.; Farmaco Sci. 30, 704 (1975)
- QSAR Modeling of Antimycobacterial Activity and Activity Against Other Bacteria of 3-Formyl Rifamycin SV Derivatives: D. Dimov, et al.; Quant. Struct.-Act. Relat. 20, 298 (2001)
