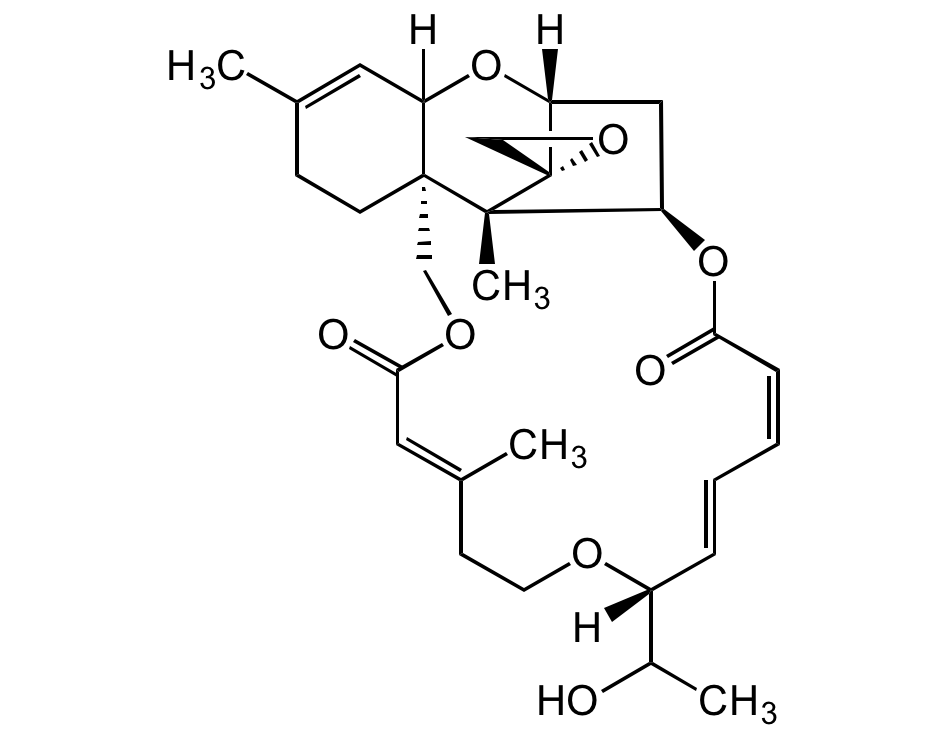
Chemical Structure
Roridin E [16891-85-3]

AG-CN2-0176
Overview
- SupplierAdipoGen Life Sciences
- Product NameRoridin E [16891-85-3]
- Delivery Days Customer10
- CAS Number16891-85-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC29H38O5
- Molecular Weight514.6
- Scientific DescriptionChemical. CAS: 16891-85-3. Formula: C29H38O5. MW: 514.6. Isolated from fungus Trichoderma sp. Mycotoxin. Implicated in human and animal toxicosis. Potent cytotoxic and antiproliferative agent against cancer cell lines. Potent antimalarial agent. Antifungal, antibiotic, phototoxic and cytostatic agent. Antiviral against arenavirus Junin (JUNV). - Mycotoxin. Implicated in human and animal toxicosis. Potent cytotoxic and antiproliferative agent against cancer cell lines. Potent antimalarial agent. Antifungal, antibiotic, phototoxic and cytostatic agent. Antiviral against arenavirus Junin (JUNV).
- SMILES[H]C12[C@@]3([C@]4(C)[C@]5(CO5)[C@](C[C@H]4OC(/C=C\C=C\[C@](OCC/C(C)=C/C(OC3)=O)([H])C(O)C)=O)([H])O2)CCC(C)=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Structure of the antibiotic Roridin E: P. Traxler, et al.; Helv. Chim. Acta 53, 2071 (1970)
- Effects of macrocyclic trichothecene mycotoxins on the murine immune system: B.J. Hughes, et al.; Arch. Environ. Contam. Toxicol. 18, 388 (1989)
- Antimalarial activity of macrocyclic trichothecenes isolated from the fungus Myrothecium verrucaria: M. Isaka, et al.; J. Nat. Prod. 62, 329 (1999)
- Phytotoxicity and mammalian cytotoxicity of macrocyclic trichothecene mycotoxins from Myrothecium verrucaria: H.K. Abbas, et al.; Phytochem. 59, 309 (2002)
- Evaluation of the antiviral activity against Junin virus of macrocyclic trichothecenes produced by the hypocrealean epibiont of Baccharis coridifolia: C.C. Garcia, et al.; Planta Med. 68, 209 (2002)
- 12'-Hydroxyl group remarkably reduces Roridin E cytotoxicity: T. Oda, et al.; Mycosciences 51, 317 (2010)
- Isolation and characterization of roridin E: C.D. Ridge, et al.; MRC 55, 337 (2017)
- Preparative separation and purification of trichothecene mycotoxins from the marine fungus Fusarium sp. LS68 by high-speed countercurrent chromatography in stepwise elution mode: Y. Liu, et al.; Mar. Drugs 16, 73 (2018)
