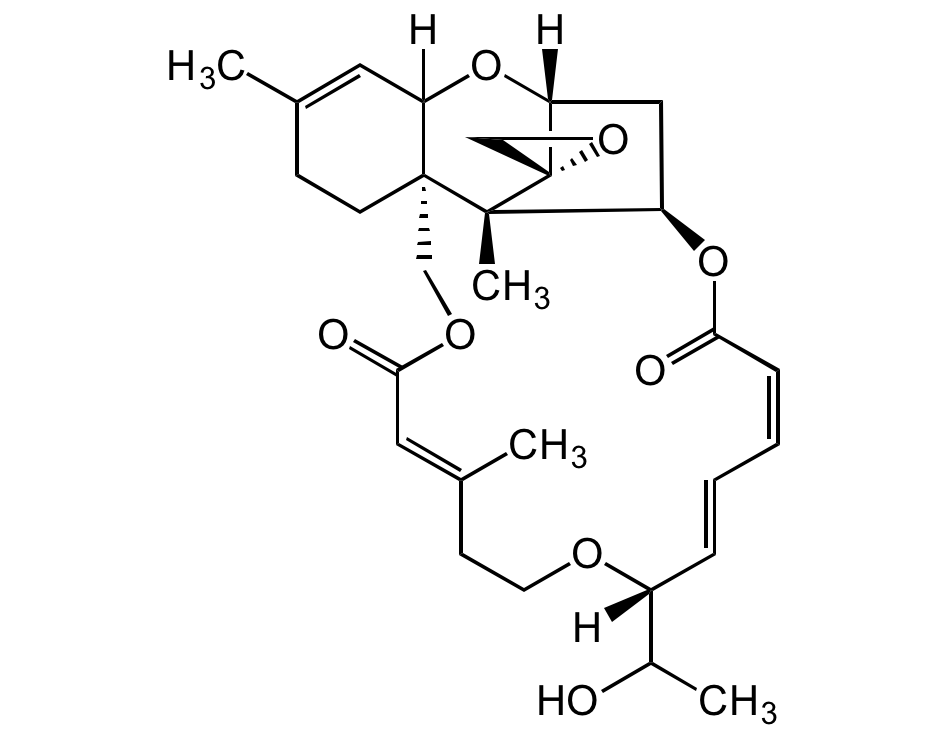
Chemical Structure
Roridin E [16891-85-3] [16891-85-3]
AG-CN2-0176
CAS Number16891-85-3
Product group Chemicals
Estimated Purity>95%
Molecular Weight514.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameRoridin E [16891-85-3] [16891-85-3]
- Delivery Days Customer10
- CAS Number16891-85-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC29H38O5
- Molecular Weight514.6
- Scientific DescriptionChemical. CAS: 16891-85-3. Formula: C29H38O5. MW: 514.6. Isolated from fungus Trichoderma sp. Mycotoxin. Implicated in human and animal toxicosis. Potent cytotoxic and antiproliferative agent against cancer cell lines. Potent antimalarial agent. Antifungal, antibiotic, phototoxic and cytostatic agent. Antiviral against arenavirus Junin (JUNV). - Mycotoxin. Implicated in human and animal toxicosis. Potent cytotoxic and antiproliferative agent against cancer cell lines. Potent antimalarial agent. Antifungal, antibiotic, phototoxic and cytostatic agent. Antiviral against arenavirus Junin (JUNV).
- SMILES[H]C12[C@@]3([C@]4(C)[C@]5(CO5)[C@](C[C@H]4OC(/C=C\C=C\[C@](OCC/C(C)=C/C(OC3)=O)([H])C(O)C)=O)([H])O2)CCC(C)=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Roridin E [16891-85-3] [16891-85-3]](https://www.targetmol.com/group3/M00/36/AE/CgoaEGayQiyEVS4oAAAAAJ0XBGc760.png)