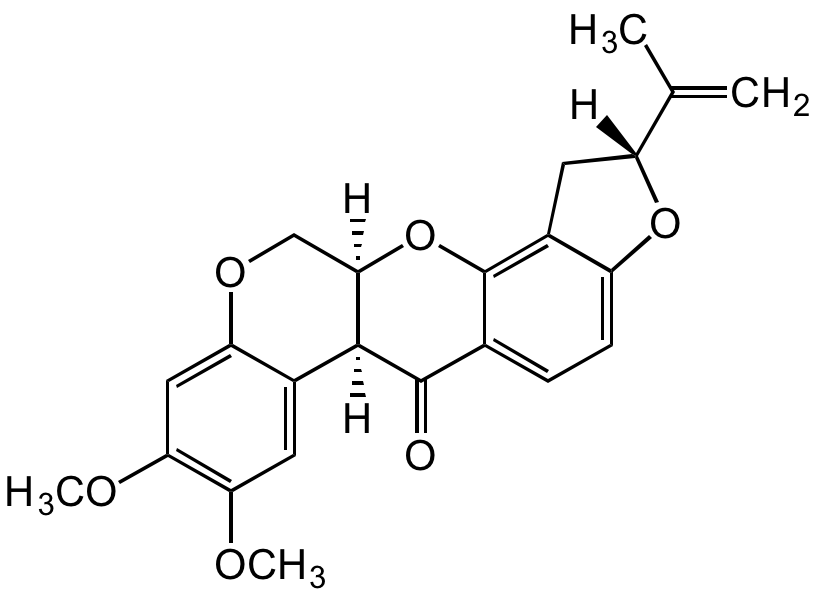
Chemical Structure
Rotenone [83-79-4]

AG-CN2-0516
Overview
- SupplierAdipoGen Life Sciences
- Product NameRotenone [83-79-4]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number83-79-4
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC23H22O6
- Molecular Weight394.4
- Scientific DescriptionCell permeable reversible and competitive mitochondrial electron transport chain complex I (NADH-CoQ reductase) inhibitor (IC50=1.7-2.2microM). Inhibits NADH/DB oxidoreductase and NADH oxidase and consequently oxidative phosphorylation (OXPHOS). Specifically inhibits NAD-linked substrate oxidation of NADH dehydrogenase. Useful agent for immunometabolism research. Inhibition of electron transport chain in mitochondria leads to blocking of the transfer of electrons from iron-sulfur centers in complex I to ubiquinone. This interferes with NADH during the creation of usable cellular energy (ATP), and Complex I is unable to pass off its electron to CoQ, creating a back-up of electrons within the mitochondrial matrix. Cellular oxygen is reduced to the radical, creating reactive oxygen species, which can damage DNA and other components of the mitochondria. Shown to inhibit mammalian cell proliferation, via suppressing microtubule assembly by binding to tubulin and inhibiting autophagy induction, by blocking lysosomal degradation of autophagic vacuoles. Shown to induce cell cycle arrest and apoptosis through production of mitochondrial ROS, consequently leading to induction of oxidative stress. Used to induce Parkinsons diseases-like syndrome in experimental animal model. Selective priming signal for NLRP3 inflammasome activation in combination with ATP, but not with Nigericin or MSU. Commonly used as a broad spectrum insecticide, piscicide and pesticide. - Chemical. CAS: 83-79-4. Formula: C23H22O6. MW: 394.4. Synthetic. Originally isolated from Lonchocarpus nicou. Cell-permeable reversible and competitive mitochondrial electron transport chain complex I (NADH-CoQ reductase) inhibitor (IC50=1.7-2.2microM). Inhibits NADH/DB oxidoreductase and NADH oxidase and consequently oxidative phosphorylation (OXPHOS). Specifically inhibits NAD-linked substrate oxidation of NADH dehydrogenase. Useful agent for immunometabolism research. Inhibition of electron transport chain in mitochondria leads to blocking of the transfer of electrons from iron-sulfur centers in complex I to ubiquinone. This interferes with NADH during the creation of usable cellular energy (ATP), and Complex I is unable to pass off its electron to CoQ, creating a back-up of electrons within the mitochondrial matrix. Cellular oxygen is reduced to the radical, creating reactive oxygen species, which can damage DNA and other components of the mitochondria. Shown to inhibit mammalian cell proliferation, via suppressing microtubule assembly by binding to tubulin and inhibiting autophagy induction, by blocking lysosomal degradation of autophagic vacuoles. Shown to induce cell cycle arrest and apoptosis through production of mitochondrial ROS, consequently leading to induction of oxidative stress. Used to induce Parkinsons diseases-like syndrome in experimental animal model. Selective priming signal for NLRP3 inflammasome activation in combination with ATP, but not with Nigericin or MSU. Commonly used as a broad spectrum insecticide, piscicide and pesticide.
- SMILESCC([C@@]1([H])CC2=C(O[C@]3([H])COC4=CC(OC)=C(OC)C=C4[C@]3([H])C5=O)C5=CC=C2O1)=C
- Storage Instruction-20°C,2°C to 8°C
- UN Number2811
- UNSPSC12352200
References
- Rotenone, an anticarcinogen, inhibits cellular proliferation but not peroxisome proliferation in mouse liver: M.L. Cunningham, et al.; Cancer Lett. 95, 93 (1995)
- Rotenone-induced G2/M cell cycle arrest and apoptosis in a human B lymphoma cell line PW: J.S. Armstrong, et al.; BBRC 289, 973 (2001)
- Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production: N. Li, et al.; J. Biol. Chem. 278, 8516 (2003)
- Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration: K. Radad, et al.; Neurochem. Int. 49, 379 (2006)
- Rotenone inhibits mammalian cell proliferation by inhibiting microtubule assembly through tubulin binding: P. Srivastava & D. Panda; FEBS J. 274, 4788 (2007)
- Pesticides and impairment of mitochondrial function in relation with the parkinsonian syndrome: C. Gomez, et al.; Front. Biosci. 12, 1079 (2007)
- Mechanisms of rotenone-induced proteasome inhibition : A.P. Chou, et al.; Neurotoxicol. 31, 367 (2010)
- Rotenone inhibits autophagic flux prior to inducing cell death: B.J. Mader, et al.; ACS Chem. Neurosci. 3, 1063 (2012)
- Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson's disease models: N. Xiong, et al.; Crit. Rev. Toxicol. 42, 613 (2012) (Review)
- Rotenone-induced oxidative stress and apoptosis in human liver HepG2 cells: M.A. Siddiqui, et al.; Mol. Cell Biochem. 384, 59 (2013)
