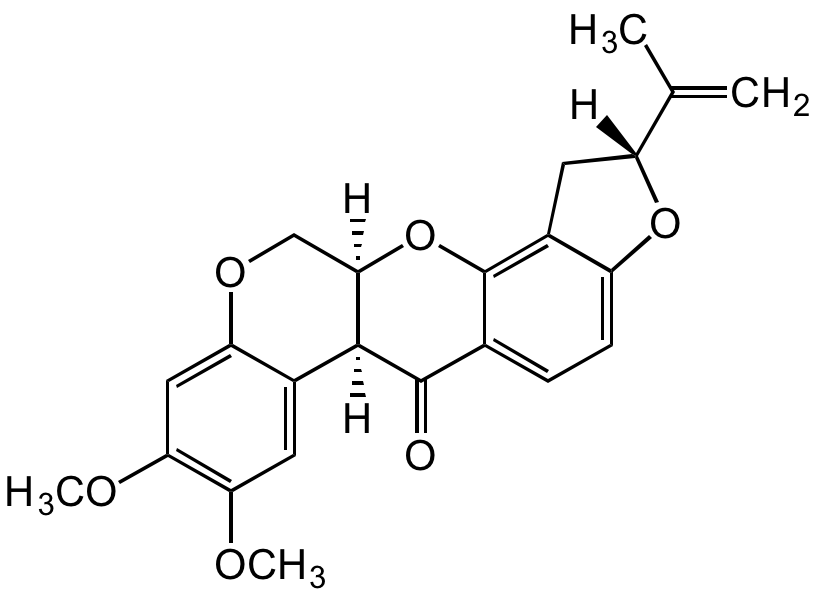
Chemical Structure
Rotenone [83-79-4] [83-79-4]
AG-CN2-0516
CAS Number83-79-4
Product group Chemicals
Estimated Purity>97%
Molecular Weight394.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameRotenone [83-79-4] [83-79-4]
- Delivery Days Customer10
- CAS Number83-79-4
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC23H22O6
- Molecular Weight394.4
- Scientific DescriptionCell permeable reversible and competitive mitochondrial electron transport chain complex I (NADH-CoQ reductase) inhibitor (IC50=1.7-2.2microM). Inhibits NADH/DB oxidoreductase and NADH oxidase and consequently oxidative phosphorylation (OXPHOS). Specifically inhibits NAD-linked substrate oxidation of NADH dehydrogenase. Useful agent for immunometabolism research. Inhibition of electron transport chain in mitochondria leads to blocking of the transfer of electrons from iron-sulfur centers in complex I to ubiquinone. This interferes with NADH during the creation of usable cellular energy (ATP), and Complex I is unable to pass off its electron to CoQ, creating a back-up of electrons within the mitochondrial matrix. Cellular oxygen is reduced to the radical, creating reactive oxygen species, which can damage DNA and other components of the mitochondria. Shown to inhibit mammalian cell proliferation, via suppressing microtubule assembly by binding to tubulin and inhibiting autophagy induction, by blocking lysosomal degradation of autophagic vacuoles. Shown to induce cell cycle arrest and apoptosis through production of mitochondrial ROS, consequently leading to induction of oxidative stress. Used to induce Parkinsons diseases-like syndrome in experimental animal model. Selective priming signal for NLRP3 inflammasome activation in combination with ATP, but not with Nigericin or MSU. Commonly used as a broad spectrum insecticide, piscicide and pesticide. - Chemical. CAS: 83-79-4. Formula: C23H22O6. MW: 394.4. Synthetic. Originally isolated from Lonchocarpus nicou. Cell-permeable reversible and competitive mitochondrial electron transport chain complex I (NADH-CoQ reductase) inhibitor (IC50=1.7-2.2microM). Inhibits NADH/DB oxidoreductase and NADH oxidase and consequently oxidative phosphorylation (OXPHOS). Specifically inhibits NAD-linked substrate oxidation of NADH dehydrogenase. Useful agent for immunometabolism research. Inhibition of electron transport chain in mitochondria leads to blocking of the transfer of electrons from iron-sulfur centers in complex I to ubiquinone. This interferes with NADH during the creation of usable cellular energy (ATP), and Complex I is unable to pass off its electron to CoQ, creating a back-up of electrons within the mitochondrial matrix. Cellular oxygen is reduced to the radical, creating reactive oxygen species, which can damage DNA and other components of the mitochondria. Shown to inhibit mammalian cell proliferation, via suppressing microtubule assembly by binding to tubulin and inhibiting autophagy induction, by blocking lysosomal degradation of autophagic vacuoles. Shown to induce cell cycle arrest and apoptosis through production of mitochondrial ROS, consequently leading to induction of oxidative stress. Used to induce Parkinsons diseases-like syndrome in experimental animal model. Selective priming signal for NLRP3 inflammasome activation in combination with ATP, but not with Nigericin or MSU. Commonly used as a broad spectrum insecticide, piscicide and pesticide.
- SMILESCC([C@@]1([H])CC2=C(O[C@]3([H])COC4=CC(OC)=C(OC)C=C4[C@]3([H])C5=O)C5=CC=C2O1)=C
- Storage Instruction-20°C,2°C to 8°C
- UN Number2811
- UNSPSC12352200


![Rotenone [83-79-4] [83-79-4]](https://www.targetmol.com/group3/M00/02/52/CgoaEGY7L8CEMJGDAAAAAMPdI-w433.png)