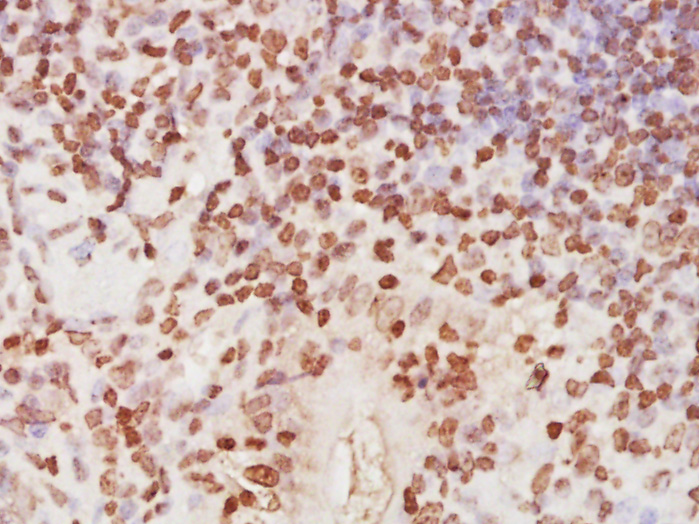
![IHC-P analysis of human tonsil tissue using GTX22175 RPA32 antibody [9H8]. IHC-P analysis of human tonsil tissue using GTX22175 RPA32 antibody [9H8].](https://www.genetex.com/upload/website/prouct_img/normal/GTX22175/GTX22175_20191203_IHC-P_94_w_23060620_908.webp)
IHC-P analysis of human tonsil tissue using GTX22175 RPA32 antibody [9H8].
RPA32 antibody [9H8]
GTX22175
ApplicationsImmunoFluorescence, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
Product group Antibodies
ReactivityHuman, Monkey
TargetRPA2
Overview
- SupplierGeneTex
- Product NameRPA32 antibody [9H8]
- Delivery Days Customer9
- Application Supplier NoteIHC-P: 1:50-1:100. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoFluorescence, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone ID9H8
- ConjugateUnconjugated
- Gene ID6118
- Target nameRPA2
- Target descriptionreplication protein A2
- Target synonymsREPA2, RP-A p32, RP-A p34, RPA32, replication protein A 32 kDa subunit, RF-A protein 2, replication factor A protein 2, replication protein A 34 kDa subunit
- HostMouse
- IsotypeIgG1
- Protein IDP15927
- Protein NameReplication protein A 32 kDa subunit
- Scientific DescriptionThis gene encodes a subunit of the heterotrimeric Replication Protein A (RPA) complex, which binds to single-stranded DNA (ssDNA), forming a nucleoprotein complex that plays an important role in DNA metabolism, being involved in DNA replication, repair, recombination, telomere maintenance, and co-ordinating the cellular response to DNA damage through activation of the ataxia telangiectasia and Rad3-related protein (ATR) kinase. The RPA complex protects single-stranded DNA from nucleases, prevents formation of secondary structures that would interfere with repair, and co-ordinates the recruitment and departure of different genome maintenance factors. The heterotrimeric complex has two different modes of ssDNA binding, a low-affinity and high-affinity mode, determined by which oligonucleotide/oligosaccharide-binding (OB) domains of the complex are utilized, and differing in the length of DNA bound. This subunit contains a single OB domain that participates in high-affinity DNA binding and also contains a winged helix domain at its carboxy terminus, which interacts with many genome maintenance protein. Post-translational modifications of the RPA complex also plays a role in co-ordinating different damage response pathways. [provided by RefSeq, Sep 2017]
- ReactivityHuman, Monkey
- Storage Instruction2°C to 8°C
- UNSPSC41116161
References
- Transcription and mRNA export machineries SAGA and TREX-2 maintain monoubiquitinated H2B balance required for DNA repair. Evangelista FM et al., 2018 Oct 1, J Cell BiolRead this paper
- Elevated levels of TRF2 induce telomeric ultrafine anaphase bridges and rapid telomere deletions. Nera B et al., 2015 Dec 7, Nat CommunRead this paper
- Structure of the herpes simplex virus 1 genome: manipulation of nicks and gaps can abrogate infectivity and alter the cellular DNA damage response. Smith S et al., 2014 Sep 1, J VirolRead this paper
- Quantitation of DNA double-strand break resection intermediates in human cells. Zhou Y et al., 2014 Feb, Nucleic Acids ResRead this paper
- Herpes simplex virus type 1 single strand DNA binding protein and helicase/primase complex disable cellular ATR signaling. Mohni KN et al., 2013, PLoS PathogRead this paper
- Efficient herpes simplex virus 1 replication requires cellular ATR pathway proteins. Mohni KN et al., 2013 Jan, J VirolRead this paper
- ATR and ATRIP are recruited to herpes simplex virus type 1 replication compartments even though ATR signaling is disabled. Mohni KN et al., 2010 Dec, J VirolRead this paper

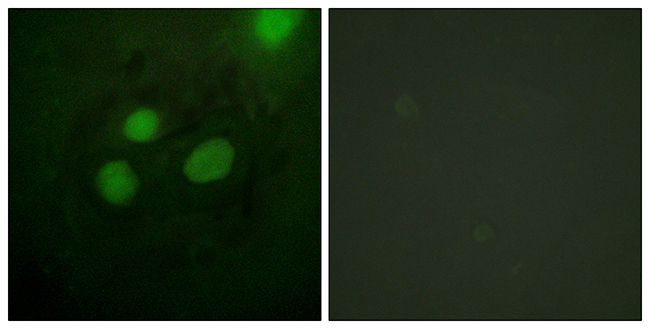



![ICC/IF analysis of C6 cells using GTX15783 RPA32 antibody [MA34]. Cells were probed without (right) or with(left) an antibody. Green : Primary antibody Blue : Nuclei Red : Actin Fixation : formaldehyde Dilution : 1:200 overnight at 4oC](https://www.genetex.com/upload/website/prouct_img/normal/GTX15783/GTX15783_314_ICC-IF_w_23060620_194.webp)
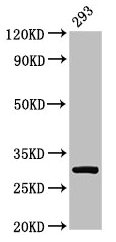
![WB analysis of various samples using GTX02702 RPA32 antibody [RPA2/3140R]. Lane 1 : MCF-7 whole cell lysate Lane 2 : T47D cell lysate](https://www.genetex.com/upload/website/prouct_img/normal/GTX02702/GTX02702_20210319_WB_w_23053122_247.webp)