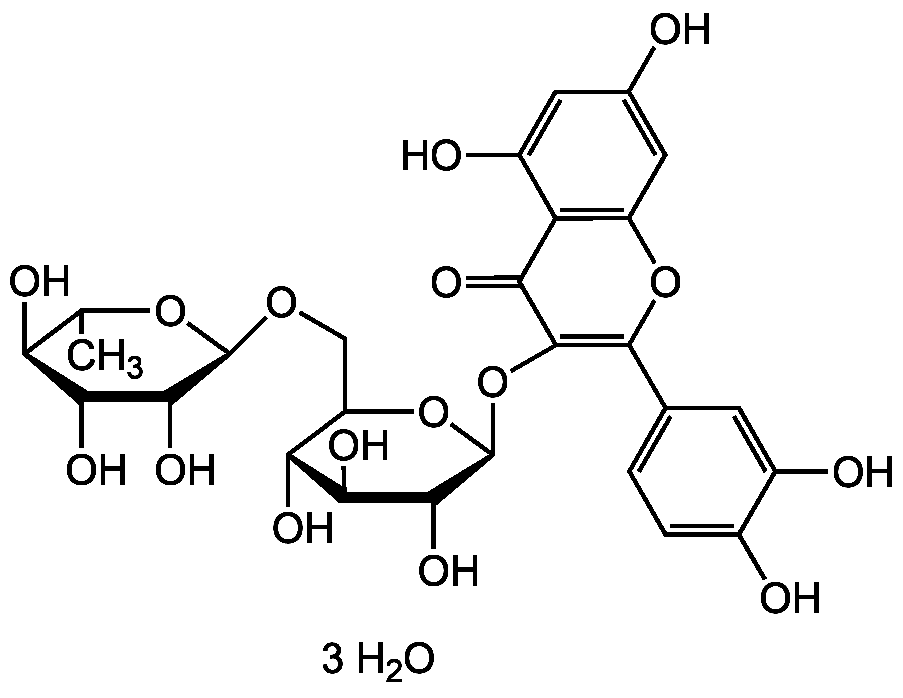
Chemical Structure
Rutin . trihydrate [250249-75-3]

AG-CN2-0408
CAS Number250249-75-3
Product group Chemicals
Estimated Purity>95%
Molecular Weight610.5 . 54.0
Overview
- SupplierAdipoGen Life Sciences
- Product NameRutin . trihydrate [250249-75-3]
- Delivery Days Customer10
- CAS Number250249-75-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC27H30O16 . 3H20
- Molecular Weight610.5 . 54.0
- Scientific DescriptionAntioxidant. Free radical scavenger. Nitric oxide (NO) scavenger [1, 5, 6]. Anticancer compound [2, 18]. Antiproliferative [10]. Apoptosis inducer [11]. Reduces induced DNA damage. Protective against carcinogenesis [3]. Hypolipidaemic. Reduces triacylglycerol levels [4]. Anti-inflammatory [5, 15]. Platelet aggregation inhibitor [7]. Anti-hyperglycaemic. Anti-adipogenic [8, 9]. Insulin-mimetic. Activates synthesis of glucose transporter GLUT4 [17]. Neuroprotective [16]. Cardioprotective. Reduces lipid peroxidation [12]. Weak alpha-glucosidase inhibitor [13]. Vasodilatory compound [14]. Brown fat activator [19]. - Chemical. CAS: 250249-75-3. Formula: C27H30O16 . 3H20. MW: 610.5 . 54.0. Isolated from Ruta graveolens. Antioxidant. Free radical scavenger. Nitric oxide (NO) scavenger. Anticancer compound. Antiproliferative. Apoptosis inducer. Reduces induced DNA damage. Protective against carcinogenesis. Hypolipidaemic. Reduces triacylglycerol levels. Anti-inflammatory. Platelet aggregation inhibitor. Anti-hyperglycaemic. Anti-adipogenic. Insulin-mimetic. Activates synthesis of glucose transporter GLUT4. Neuroprotective. Cardioprotective. Reduces lipid peroxidation. Weak alpha-glucosidase inhibitor. Vasodilatory compound.
- SMILESCC1O[C@@H](OCC2O[C@@H](OC3=C(OC4=C(C(O)=CC(O)=C4)C3=O)C3=CC=C(O)C(O)=C3)C(O)[C@H](O)[C@@H]2O)C(O)[C@@H](O)[C@H]1O
- Storage Instruction2°C to 8°C
- UNSPSC12352200
References
- Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation: I.B. Afanas'ev, et al.; Biochem. Pharmacol. 38, 1763 (1989)
- Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia: E.E. Deschner, et al.; Carcinogenesis 12, 1193 (1991)
- Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats: R.P. Webster, et al.; Cancer Lett. 109, 185 (1996)
- Hypolipidaemic effects of naringenin, rutin, nicotinic acid and their associations: K.F. Santos, et al.; Pharmacol. Res. 40, 493 (1999)
- Oxidative stress in rheumatoid arthritis leukocytes: suppression by rutin and other antioxidants and chelators: E.A. Ostrakhovitch & I.B. Afanas'ev; Biochem. Pharmacol. 62, 743 (2001)
- In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E(2) production: S.C. Shen, et al.; Eur. J. Pharmacol. 446, 187 (2002)
- Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets: J.R. Shen et al.; J. Agric. Food Chem. 52, 4414 (2004)
- Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats: N. Kamalakkannan & P.S. Prince; Basic Clin. Pharmacol. Toxicol. 98, 97 (2006)
- Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet: I. Choi, et al.; Biofactors 26, 273 (2006)
- Rutin inhibits the proliferation of murine leukemia WEHI-3 cells in vivo and promotes immune response in vivo: J.P. Lin, et al.; Leuk. Res. 33, 823 (2009)
