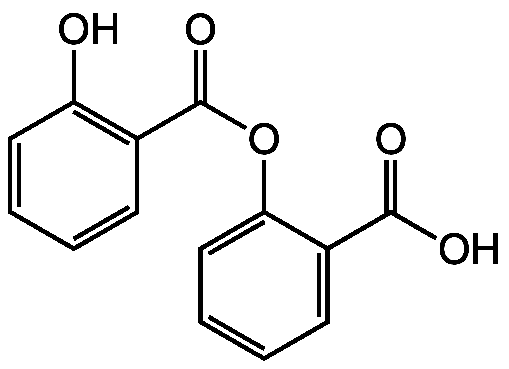
Chemical Structure
Salsalate [552-94-3]

AG-CR1-3574
CAS Number552-94-3
Product group Chemicals
Estimated Purity>98%
Molecular Weight258.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameSalsalate [552-94-3]
- Delivery Days Customer10
- ADR Class9
- CAS Number552-94-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC14H10O5
- Molecular Weight258.2
- Scientific DescriptionChemical. CAS: 552-94-3. Formula: C14H10O5. MW: 258.2. Nonacetylated salicylate. Non-steroidal anti-inflammatory drug (NSAID). Reduces pain and inflammation caused by conditions such as rheumatoid arthritis, osteoarthritis and related rheumatic conditions. Prostaglandin synthesis inhibitor in vivo. Inactivates cyclooxygenase-1 (COX-1) and -2 (COX-2). IKKbeta/NF-kappaB inhibitor (at significantly higher concentrations than required for COX inhibition). Used to target inflammation in the treatment of insulin resistance, type 2 diabetes, or rheumatic pain. It reduces blood glucose concentrations in patients with type 2 diabetes, as well as in insulin-resistant patients without diabetes. Reduces blood glucose, triglyceride, free fatty acid and C-reactive protein concentrations, improves glucose utilization and increases circulating insulin and adiponectin concentrations in obese adults at risk for the development of type 2 diabetes as well as for patients with type 2 diabetes. Dimeric prodrug comprising two esterified salicylate moieties. It is advantageous over sodium salicylate because it is insoluble at the acid pH of the stomach and passes suspended but undissolved into the small intestine, sparing the gastric mucosa direct contact. - Nonacetylated salicylate. Non-steroidal anti-inflammatory drug (NSAID). Reduces pain and inflammation caused by conditions such as rheumatoid arthritis, osteoarthritis and related rheumatic conditions. Prostaglandin synthesis inhibitor in vivo. Inactivates cyclooxygenase-1 (COX-1) and -2 (COX-2). IKKbeta/NF-kappaB inhibitor (at significantly higher concentrations than required for COX inhibition). Used to target inflammation in the treatment of insulin resistance, type 2 diabetes, or rheumatic pain. It reduces blood glucose concentrations in patients with type 2 diabetes, as well as in insulin-resistant patients without diabetes. Reduces blood glucose, triglyceride, free fatty acid and C-reactive protein concentrations, improves glucose utilization and increases circulating insulin and adiponectin concentrations in obese adults at risk for the development of type 2 diabetes as well as for patients with type 2 diabetes. Dimeric prodrug comprising two esterified salicylate moieties. It is advantageous over sodium salicylate because it is insoluble at the acid pH of the stomach and passes suspended but undissolved into the small intestine, sparing the gastric mucosa direct contact.
- SMILESOC(=O)C1=CC=CC=C1OC(=O)C1=C(O)C=CC=C1
- Storage Instruction2°C to 8°C,-20°C
- UN NumberUN 3077
- UNSPSC12352200
References
- Reduced risk of NSAID gastropathy (GI mucosal toxicity) with nonacetylated salicylate (salsalate): an endoscopic study: S. Roth, et al.; Semin. Arth. Rheum. 19, 11 (1990)
- Inhibition of NF-kappa B by sodium salicylate and aspirin: E. Kopp & S. Ghosh; Science 265, 956 (1994)
- Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration: J.W. Pierce, et al.; J. Immunol. 156, 3961 (1996)
- The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta: M.J. Yin, et al.; Nature 396, 77 (1998)
- Salsalate improves glycemia and inflammatory parameters in obese young adults: A. Fleischman, et al.; Diabetes Care 31, 289 (2008)
- Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes: A.B. Goldfine, et al.; Clin. Transl. Sci. 1, 36 (2008)
- The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised double-blind placebo-controlled study: J. Koska, et al.; Diabetologia 52, 385 (2009)
- Nuclear factor-kappaB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans: G.L. Pierce, et al.; Circulation 119, 1284 (2009)
- The effects of salsalate on glycemic control in patients with type 2 diabetes: A randomized trial: A.B. Goldfine, et al.; Ann. Intern. Med. 152, 346 (2010)
- Potential role of salicylates in type 2 diabetes: M.M. Rumore & K.S. Kim; Ann. Pharmacother. 44, 1207 (2010) (Review)
