Chemical Structure
SB415286
AG-CR1-3658
Overview
- SupplierAdipoGen Life Sciences
- Product NameSB415286
- Delivery Days Customer10
- CAS Number264218-23-7
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationWarning
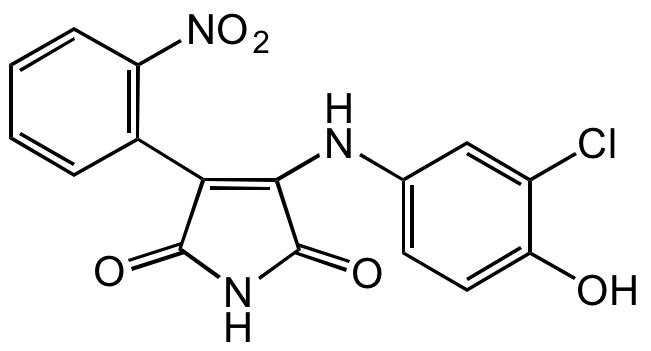
- Molecular FormulaC16H10ClN3O5
- Molecular Weight359.7
- Scientific DescriptionChemical. CAS: 264218-23-7. Formula: C16H10ClN3O5. MW: 359.7. Synthetic. Potent and selective cell permeable, ATP-competitive inhibitor of GSK3alpha with an IC50 value of 78nM (and similar potency for GSK3beta). DYRK1A inhibitor (IC50=0.9microM). Stimulates glycogen synthesis in liver cells and induces beta-catenin-dependent gene transcription. Acts as neuroprotectant and prevents neuronal cell death induced by PI3-kinase pathway. Anticancer agent with antiproliferative activity. Glycogen synthase kinase 3 (GSK3) is a serine/threonine protein kinase that is inhibited by an assortment of extracellular stimuli such as insulin, growth factors, cell specification factors and cell adhesion. Its activity regulates many cell functions including the control of cell division, apoptosis and inflammation. - Potent and selective cell permeable, ATP-competitive inhibitor of GSK3alpha with an IC50 value of 78nM (and similar potency for GSK3beta). DYRK1A inhibitor (IC50=0.9microM). Stimulates glycogen synthesis in liver cells and induces beta-catenin-dependent gene transcription. Acts as neuroprotectant and prevents neuronal cell death induced by PI3-kinase pathway. Anticancer agent with antiproliferative activity. Glycogen synthase kinase 3 (GSK3) is a serine/threonine protein kinase that is inhibited by an assortment of extracellular stimuli such as insulin, growth factors, cell specification factors and cell adhesion. Its activity regulates many cell functions including the control of cell division, apoptosis and inflammation.
- SMILESClC1=CC(NC2=C(C3=C([N+]([O-])=O)C=CC=C3)C(NC2=O)=O)=CC=C1O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC51202000

![SB 415286 [264218-23-7]](https://www.targetmol.com/group3/M00/35/76/CgoaEGayH9-EUKWWAAAAABFsps4550.png)
