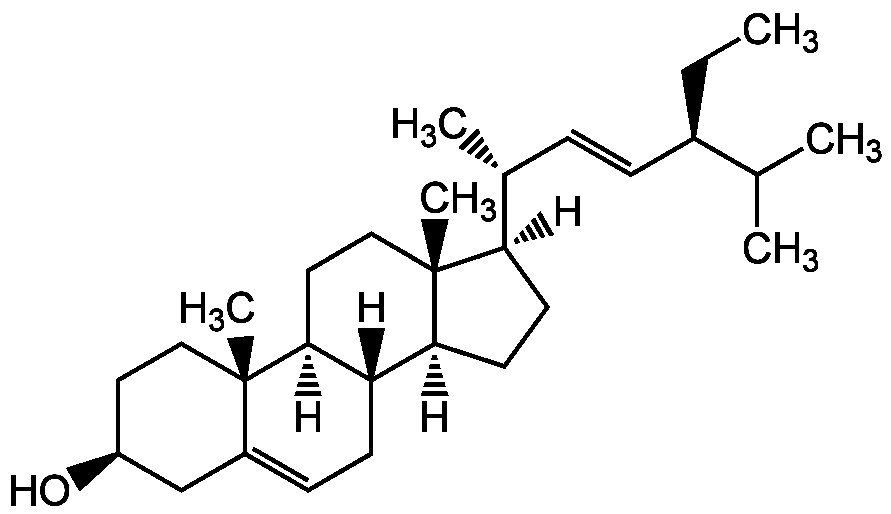
Chemical Structure
Stigmasterol [83-48-7]

AG-CN2-0412
Overview
- SupplierAdipoGen Life Sciences
- Product NameStigmasterol [83-48-7]
- Delivery Days Customer10
- CAS Number83-48-7
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC29H48O
- Molecular Weight412.7
- Scientific DescriptionAnti-hypercholestrolemic compound [1, 7]. Anti-inflammatory and immune-modulating effects [2, 4]. Anticancer compound. Chemopreventive [3, 12, 13]. Cytostatic. Cell growth inhibitor [5]. Antimutagenic [6]. Potent in vitro antagonist of FXR (farnesoid X receptor) [8]. DNA polymerase beta inhibitor [9]. Potent antioxidant, hypoglycemic and thyroid inhibiting agent [10]. Anti-osteoarthritic. Decreases the expression of matrix metalloproteinases [11]. Neuroprotective [15]. Is used as a precursor for synthetic progesterone and vitamin D3 and is an intermediate in the biosynthesis of androgens, estrogens and corticoids [14]. - Chemical. CAS: 83-48-7. Formula: C29H48O. MW: 412.7. Synthetic. Originally isolated from various plants and marine organisms. Anti-hypercholestrolemic compound. Anti-inflammatory and immune-modulating effects. Anticancer compound. Chemopreventive. Cytostatic. Cell growth inhibitor. Antimutagenic. Potent in vitro antagonist of FXR (farnesoid X receptor). DNA polymerase beta inhibitor. Potent antioxidant, hypoglycemic and thyroid inhibiting agent. Anti-osteoarthritic. Decreases the expression of matrix metalloproteinases. Neuroprotective. Is used as a precursor for synthetic progesterone and vitamin D3 and is an intermediate in the biosynthesis of androgens, estrogens and corticoids.
- SMILES[H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)\C=C\[C@@H](CC)C(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Antihypercholesterolemic studies with sterols: beta-sitosterol and stigmasterol: R.F. Chandler, et al.; J. Pharm. Sci. 68, 245 (1979)
- Topical antiinflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models: M.D. Garcia, et al.; Phytother. Res. 13, 78 (1999)
- Phytosterols as anticancer dietary components: evidence and mechanism of action: A.B. Awad & C.S. Fink; J. Nutr. 130, 2127 (2000) (Review)
- Anti-Inflammatory and Immunomodulating Properties of a Sterol Fraction from Sideritis foetens CLEM: N. Antonio, et al.; Biol. Pharm. Bull. 24, 470 (2001)
- Cyostatic activity of Achillea ageratum L.: M.A. Gomez, et al.; Phytother. Res. 15, 633 (2001)
- Antimutagenic Constituents from the Thorns of Gleditsia sinensis: L. Jae-Chul, et al.; Chem. Pharm. Bull. 53, 561 (2005)
- Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat: A.K. Batta, et al.; Metabolism 55, 292 (2006)
- Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR: B.A. Carter, et al.; Pediatr. Res. 62, 301 (2007)
- Inhibitors of DNA polymerase beta: Activity and mechanism: G. Zhijie, et al.; Bioorg. Med. Chem. 16, 4331 (2008)
- Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma: S. Panda, et al.; Fitoterapia 80, 123 (2009)
