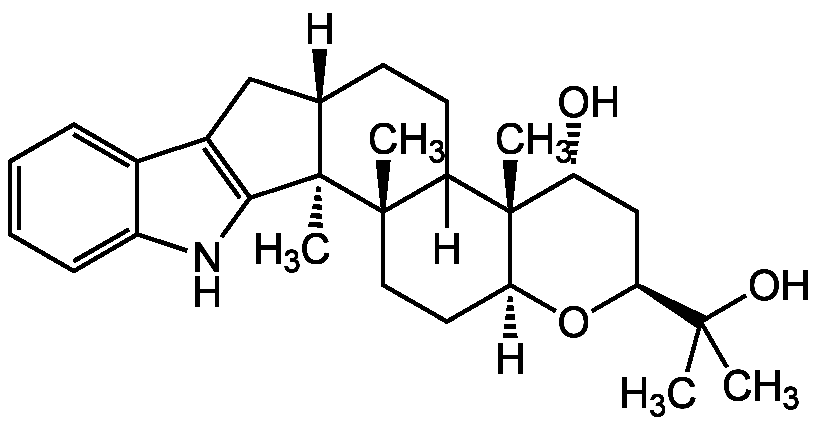
Chemical Structure
Terpendole E [167427-23-8]

AG-CN2-0127
Overview
- SupplierAdipoGen Life Sciences
- Product NameTerpendole E [167427-23-8]
- Delivery Days Customer10
- CAS Number167427-23-8
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC28H39NO3
- Molecular Weight437.6
- Scientific DescriptionAcyl-CoA:cholesterol acyltransferase (ACAT) inhibitor [1, 2]. Mitotic kinesin Eg5 (Mitotic Kinesin Spindle Protein; KSP) inhibitor [3-7]. Specific M phase inhibitor [4]. - Chemical. CAS: 167427-23-8. Formula: C28H39NO3. MW: 437.6. Isolated from Albophoma yamanashiensis. Acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor. Mitotic kinesin Eg5 (Mitotic Kinesin Spindle Protein; KSP) inhibitor. Specific M phase inhibitor.
- SMILES[H][C@]12CC3=C(NC4=CC=CC=C34)[C@]1(C)[C@@]1(C)CC[C@]3([H])O[C@@H](C[C@@H](O)[C@@]3(C)C1([H])CC2)C(C)(C)O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Microbial metabolites affecting lipid biosynthesis: S. Omura & H. Tomoda; Pure Appl. Chem. 66, 2267 (1994)
- Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. I. Production, isolation and biological properties: X.H. Huang, et al.; J. Antibiot. (Tokyo) 48, 1 (1995)
- A novel action of terpendole E on the motor activity of mitotic Kinesin Eg5: J. Nakazawa, et al.; Chem. Biol. 10, 131 (2003)
- Development and application of bioprobes for Mammalian cell cycle analyses: H. Osada; Curr. Med. Chem. 10, 727 (2003) (Review)
- Docking studies on kinesin spindle protein inhibitors: an important cooperative 'minor binding pocket' which increases the binding affinity significantly: C. Jiang, et al.; J. Mol. Model 13, 987 (2007)
- A novel approach to indoloditerpenes by Nazarov photocyclization: synthesis and biological investigations of terpendole E analogues: F. Churruca, et al.; Org. Lett. 12, 2096 (2010)
- Kinesin spindle protein (KSP) inhibitors with 2,3-fused indole scaffolds: S. Oishi, et al.; J. Med. Chem. 53, 5054 (2010)
