Chemical Structure
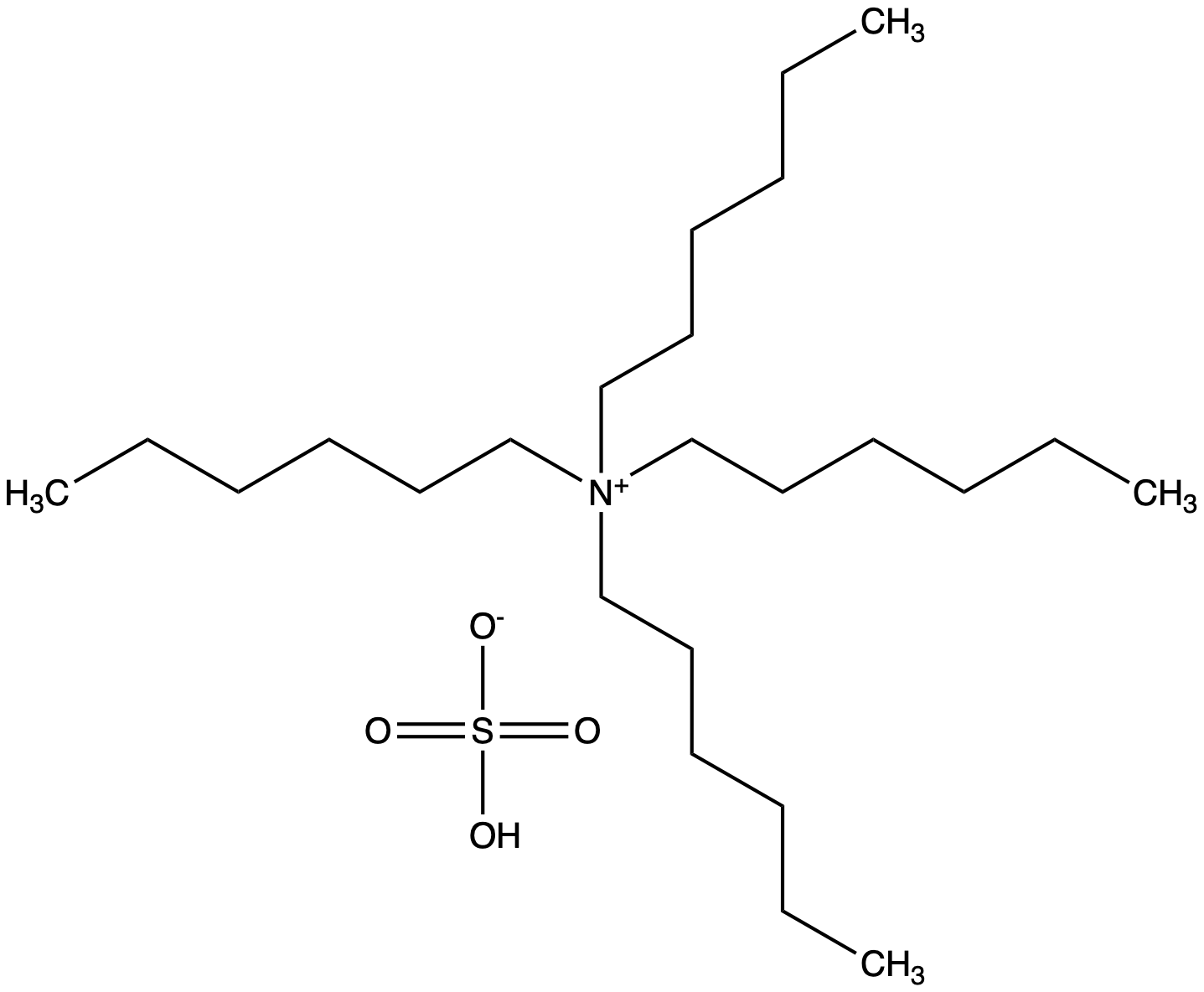
Tetrahexylammonium hydrogensulfate [32503-34-7] [32503-34-7]
CDX-T0610
CAS Number32503-34-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight451.75
Overview
- SupplierChemodex
- Product NameTetrahexylammonium hydrogensulfate [32503-34-7] [32503-34-7]
- Delivery Days Customer10
- CAS Number32503-34-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC24H53NO4S
- Molecular Weight451.75
- Scientific DescriptionChemical. CAS: 32503-34-7. Formula: C24H53NO4S. MW: 451.75. Tetrahexylammonium hydrogensulfate (THAHS) is a quaternary ammonium salt used in various chemical and biochemical applications. It consists of a tetracationic ammonium ion (C6H13)4N+ and a hydrogen sulfate anion (HSO4-). THAHS is used as a phase-transfer catalyst (PCT) and can facilitate various chemical reactions by aiding in the transfer of reactants between immiscible phases. Phase-transfer catalysis involves the transfer of reactants from one phase (usually an aqueous phase) to another phase (usually an organic phase) to facilitate a chemical reaction. THAHS can be employed in organic synthesis, particularly in reactions that involve the transfer of anions or reagents between organic and aqueous phases. Some common applications include, quaternization reactions, nucleophilic substitution reactions or halogenation reactions. It can also be used in processes that involve the extraction or separation of specific ions or compounds from one phase to another. - Tetrahexylammonium hydrogensulfate (THAHS) is a quaternary ammonium salt used in various chemical and biochemical applications. It consists of a tetracationic ammonium ion (C6H13)4N+ and a hydrogen sulfate anion (HSO4-). THAHS is used as a phase-transfer catalyst (PCT) and can facilitate various chemical reactions by aiding in the transfer of reactants between immiscible phases. Phase-transfer catalysis involves the transfer of reactants from one phase (usually an aqueous phase) to another phase (usually an organic phase) to facilitate a chemical reaction. THAHS can be employed in organic synthesis, particularly in reactions that involve the transfer of anions or reagents between organic and aqueous phases. Some common applications include, quaternization reactions, nucleophilic substitution reactions or halogenation reactions. It can also be used in processes that involve the extraction or separation of specific ions or compounds from one phase to another.
- SMILESOS([O-])(=O)=O.CCCCCC[N+](CCCCCC)(CCCCCC)CCCCCC
- Storage InstructionRT
- UNSPSC12352200
